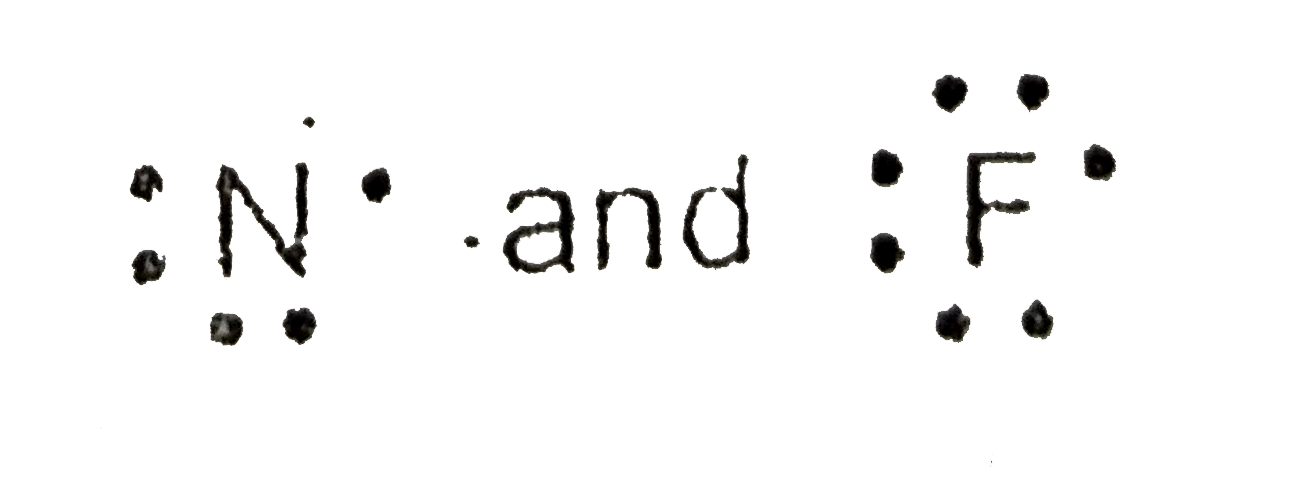Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section -A Objective Type Questions (One option is correct)|60 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section - B Objective Type Questions(One option is correct)|37 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosBIOMOLECULES
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Try Yourself
- Suppose an element X has Lewis symbol as overset(.)(X) . Identify the ...
Text Solution
|
- Draw the Lewis symbol for the following nitrogen (N) and fluorine (F)
Text Solution
|
- Draw the Lewis dot structure of hydrogen moelcule .
Text Solution
|
- Draw the Lewis dot structure of the HCl molecule .
Text Solution
|
- Draw the Lewis dot structure of Methane (CH(4)) molecule .
Text Solution
|
- Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule .
Text Solution
|
- Draw the Lewis structure of NH(4)^(+) ion .
Text Solution
|
- Write the Lewis structure of hydrogen peroxide .
Text Solution
|
- Write the Lewis dot structure of the nitrite ion (NO(2)^(Θ)) .
Text Solution
|
- Draw the Lewis structure of SOCl(2) (Thionyl chloride)
Text Solution
|
- The formal charges on the three O atoms in the O3 molecule are
Text Solution
|
- Calculate the formal charge on Cl atom in H ClO(4).
Text Solution
|
- Write is the bond order between C and C in H - C -= C-H ?
Text Solution
|
- What is the bond order between C and O in CO ?
Text Solution
|
- Explain the reasonance structure of CO(2) molecule?
Text Solution
|
- Explain the important aspects of resonance with respect to the CO(3)^(...
Text Solution
|
- Discuss the shape of the B Cl(3) molecules using VSEPR model .
Text Solution
|
- Discuss the shape of the SiF(4) molecule using VSEPR model .
Text Solution
|
- Discuss the shape of H(3)O^(+) using VSEPR model .
Text Solution
|
- Discuss the shape of RF(4)^(-) using VSEPR model
Text Solution
|