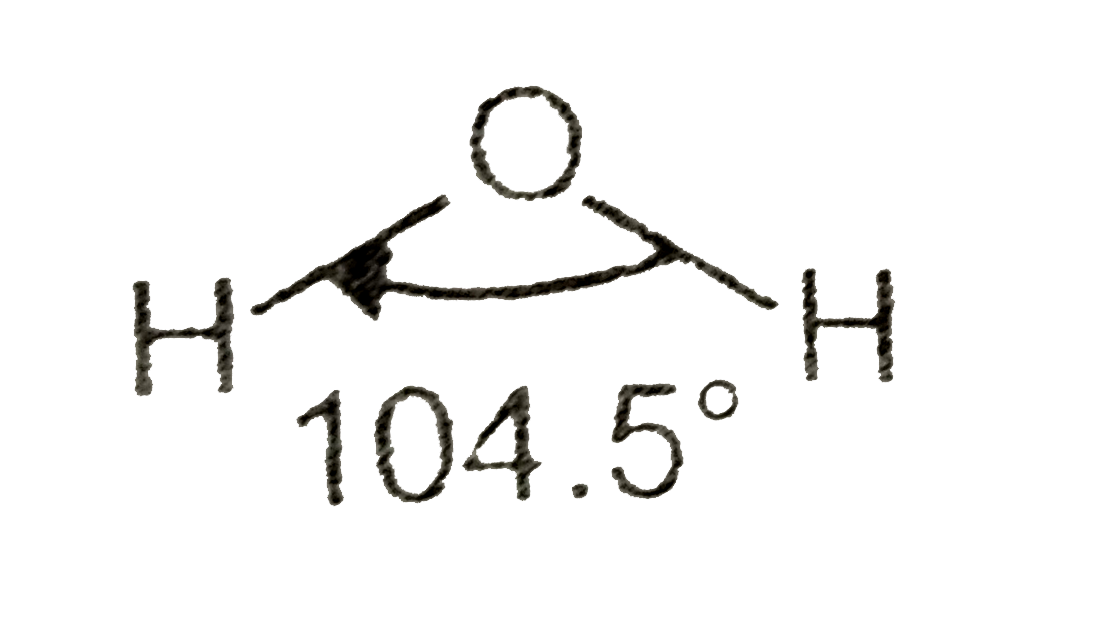
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section - E (Assertion-Reason Type Questions)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section -F (Matrix- Match Type Questions)|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section - C Objective Type Questions(More than one options are correct )|13 VideosBIOMOLECULES
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Assignment Section - D (Linked Comprehension Type Questions)
- Molecular orbital (MOT) the atoms in a molecule are supposed to loose ...
Text Solution
|
- Molecular orbital (MOT) the atoms in a molecule are supposed to loose ...
Text Solution
|
- Molecular orbital (MOT) the atoms in a molecule are supposed to loose ...
Text Solution
|
- Electric Dipole moment is a vector quantify . If a compound contain mo...
Text Solution
|
- Electric Dipole moment is a vector quantify . If a compound contain mo...
Text Solution
|
- Electric Dipole moment is a vector quantify . If a compound contain mo...
Text Solution
|
- Lattice energy : Lattice energy is the amount of energy released when ...
Text Solution
|
- Lattice energy : Lattice energy is the amount of energy released when ...
Text Solution
|
- Shape of the compound depend on type and number of electron pair aroun...
Text Solution
|
- Shape of the compound depend on type and number of electron pair aroun...
Text Solution
|
- Shape of the compound depend on type and number of electron pair aroun...
Text Solution
|