Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section - H (Multiple True-False Type Questions) Identify the correct combination of true(T) and false(F) of the given three statements.|5 VideosBIOMOLECULES
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Assignment Section- I (Subjective Type Questions)
- Why H(2) is more stable than H(2)^(+) while He(2)^(+) is more stable t...
Text Solution
|
- CO is stable , but analogous SiO is not stable . Why ?
Text Solution
|
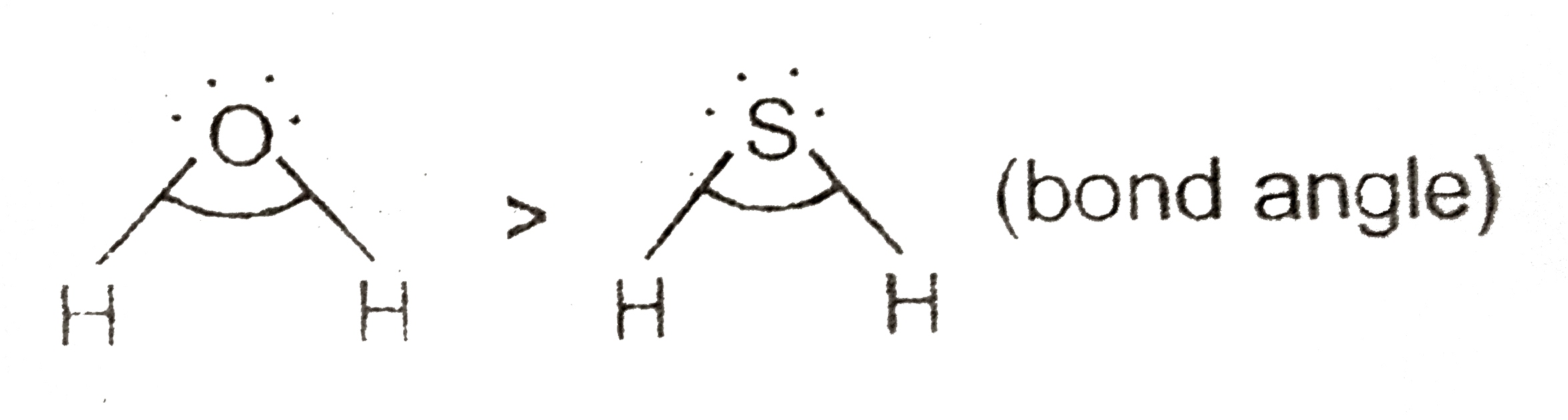
- Why H-S-H bond angle in H(2)S is smaller than H-O-H bond angle in H(2)...
Text Solution
|
- Na(2)CO(3) does not decompose on heating whereas CaCO(3) decomposes . ...
Text Solution
|
- Why Al Cl(3) is a covalent molecule but is ionized in water to give Al...
Text Solution
|
- Why is red phosphorus less reactive than white phosphorus ?
Text Solution
|
- Why carbon exists as graphite but silicon does not exist in any such f...
Text Solution
|
- Iodine in lF(7) is surrounded by 7 pairs of electrons yet it is stable...
Text Solution
|
- Calculate the percentage of covalent character of HX having bond lengt...
Text Solution
|
- Why XeF(5)^(-) is planar ?
Text Solution
|