Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
AAKASH INSTITUTE|Exercise Try Yourself|56 VideosTHE SOLID STATE
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION-A (OBJECTIVE)|50 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assignment (Section-J)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-THE SOLID STATE -Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)
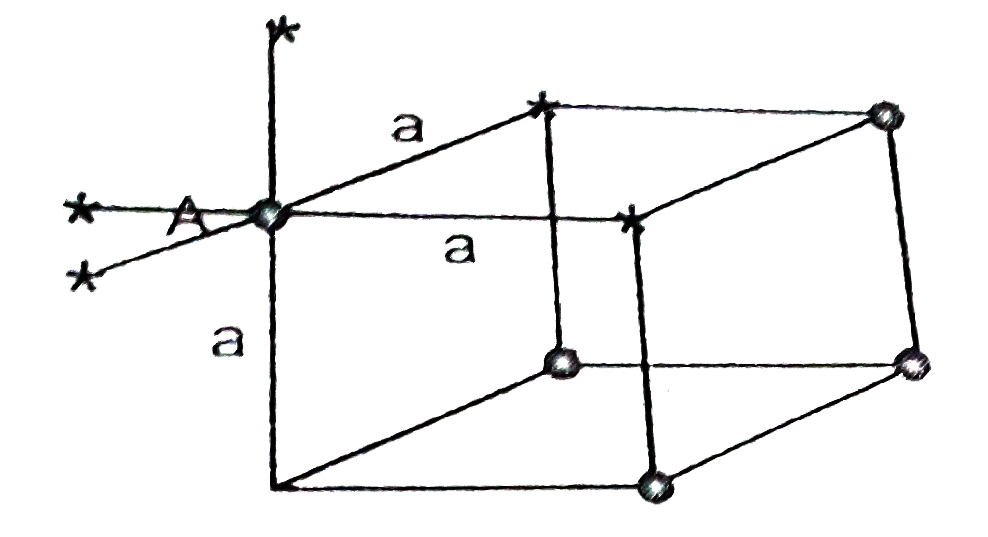
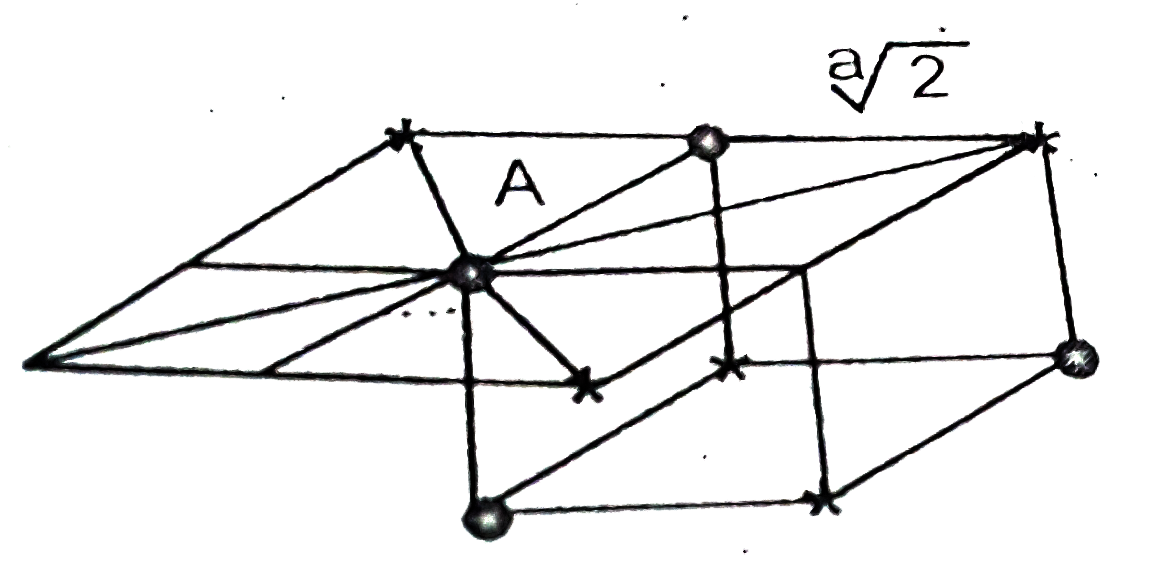
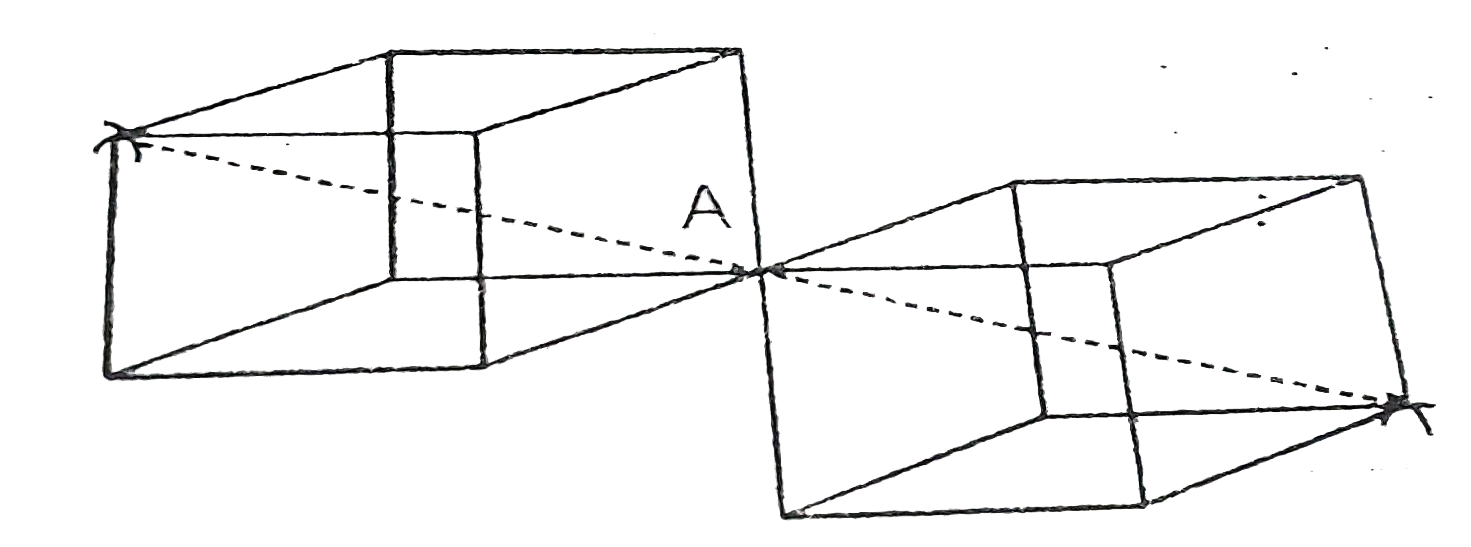
- Find out of 1st,2nd and 3rd coordination number for an atom located at...
Text Solution
|
- A : In NaCl structure, the interionic distance is a/2 (a = Unit cell e...
Text Solution
|
- A : The co - ordination number of CaF(2) is 8 : 4. R : Ca^(2+) ion...
Text Solution
|
- A : The number of spheres are equal to the number of octahedral voids ...
Text Solution
|
- A : In Schottky defect, density of crystal decreases. R : Equal nu...
Text Solution
|
- A : If a tetrad axis is passed through the unit cell of NaCl and all i...
Text Solution
|
- A : A particle at the corner of CCP unit cell has (1)/(8)th of its con...
Text Solution
|
- A : Glass belongs to the category of covalent network solid. R : U...
Text Solution
|
- A : NaCl shows Schottky defect at room temperature. R : NaCl shows...
Text Solution
|
- A : Fe(3)O(4) is ferrimagnetic at room temperature but becomes paramag...
Text Solution
|
- A : In molecular solids the lattice points are occupied by the atoms o...
Text Solution
|
- A : Silicon is insulator at 0 K but semiconductor at room temperature....
Text Solution
|
- A : Amorphous solids are isotropic. R : Amorphous solids are not rig...
Text Solution
|
- A : In NaCl coordination number of Cl^(-) ion is 6 but in CsCl coordin...
Text Solution
|
- A : All crystals of same substance possess the same elements of symmet...
Text Solution
|
- A : AgBr shows both Schottky and Frenkel defect. R : AgBr is a cry...
Text Solution
|
- A : Number of carbon atoms per unit cell in diamond is 8. R : The ...
Text Solution
|
- A : The coordination number of ionic compound depends upon radius rati...
Text Solution
|
- A : Number of rectangular plane in a cubic crystal is 3. R : Recta...
Text Solution
|
- A : ccp is more efficient than hcp. R : Packing fraction is differe...
Text Solution
|
- A : Coordination number of both Na^(+) and Cl^(-) NaCl is 6. R : Se...
Text Solution
|