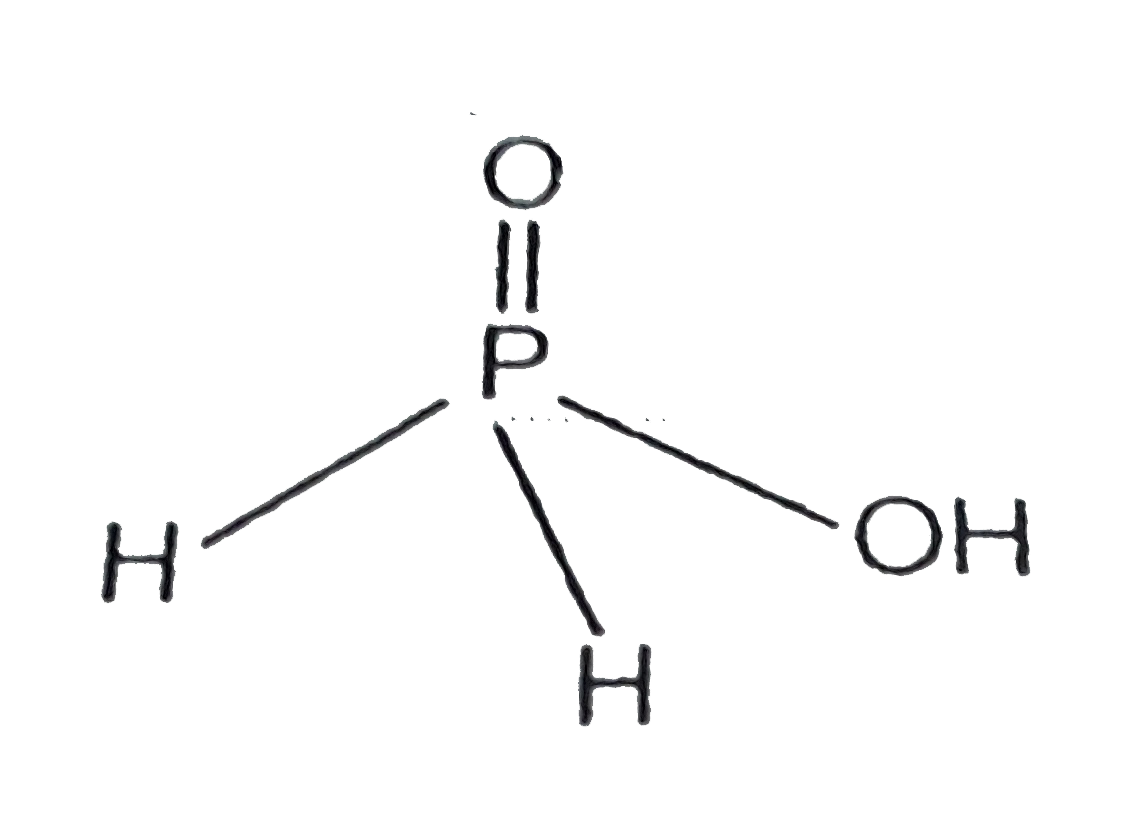
Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Try yourself|70 VideosTHE P-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assignment Section-A)|94 VideosTHE D AND F-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assingment ( Section-J Aakash Challengers Questions )|10 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assignment (Section-J)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-THE P-BLOCK ELEMENTS-Assignment Section-J)
- How do you account for reducing behaviour of H(3)PO(2) on the basis of...
Text Solution
|
- Correct among the follownig
Text Solution
|
- Incorrect among the following
Text Solution
|
- B(2)H(6) reacts with NH(3) to form
Text Solution
|
- SiC is a/an
Text Solution
|
- SiO(2) is solid while CO(2) is gas. Why?
Text Solution
|
- Chain silicates can be represented as
Text Solution
|
- Which of the following halogens can oxidise H(2)S to S?
Text Solution
|
- NH(3)+NaOCl → NH(2)-NH(2)+NH(4)Cl To obtain this product in large am...
Text Solution
|
- Which of the following oxide does not form dimer?
Text Solution
|
- Choose the correct statement regarding ice.
Text Solution
|
- Pure oxygen is colourless gas but liquid and solid O(2) is pale blue o...
Text Solution
|
- Choose the correct regarding heating of S.
Text Solution
|
- In which, degree of hydrolysis is expected to be highest?
Text Solution
|
- Choose the correct regarding bond length
Text Solution
|
- Br(2) gas is of red brown colour and I(2) gas is of violet volour. Thi...
Text Solution
|
- Which of the following halides has Cl^(-) as a bridging atom?
Text Solution
|