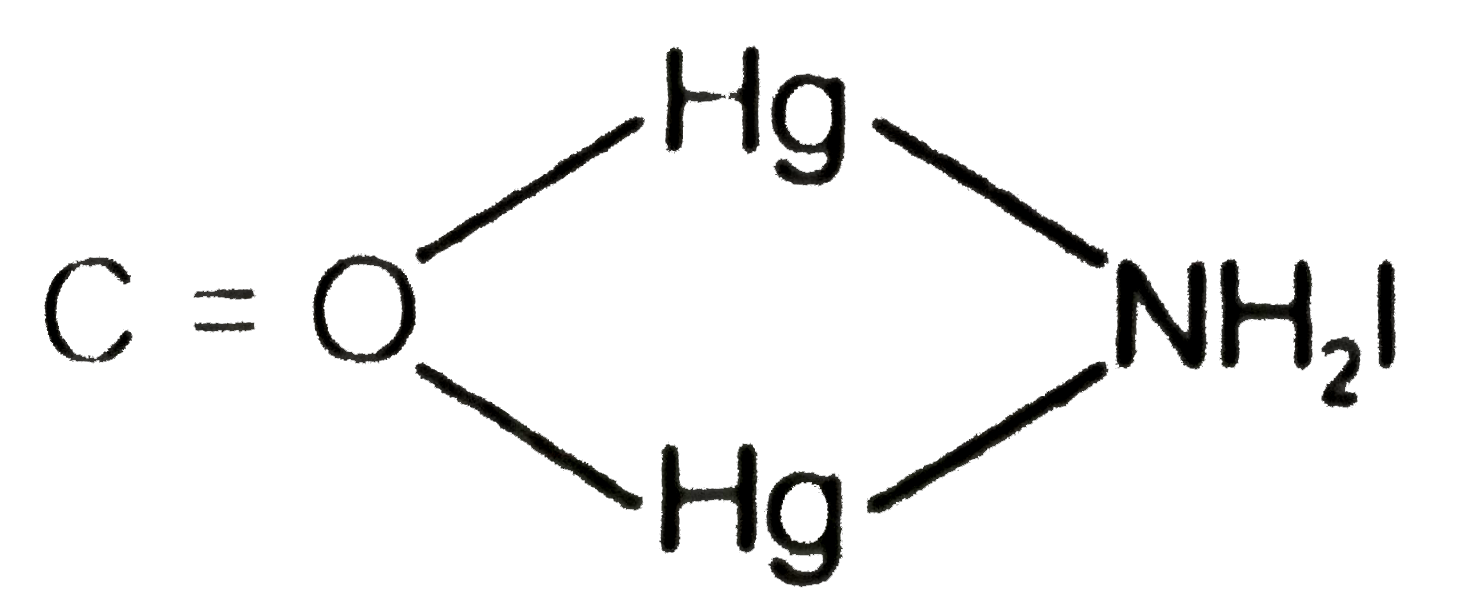
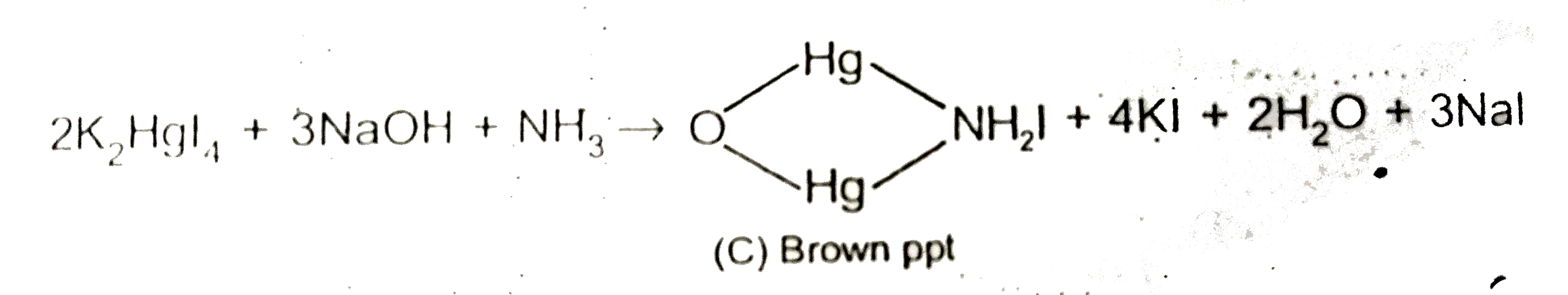
Text Solution
Verified by Experts
Topper's Solved these Questions
PRINCIPLES OF QUALITATIVE ANALYSIS
AAKASH INSTITUTE|Exercise Try Yourself|1 VideosPRINCIPLES OF QUALITATIVE ANALYSIS
AAKASH INSTITUTE|Exercise Assignment (SECTION A)|25 VideosPOLYMERS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION-D)|13 VideosREDOX REACTIONS
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - D|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-PRINCIPLES OF QUALITATIVE ANALYSIS-Assignment (SECTION H)
- A compound (A) when treated with Kl gives a Scarlet red ppt. (B) which...
Text Solution
|
- When a white powder (A) is strongly heated, it gives of a colourless, ...
Text Solution
|
- A solid laboratory reagent (A) give following reactions. (i) It impa...
Text Solution
|
- An aqueous of a gas (X) shows the following reactions : (a) It turns...
Text Solution
|
- A black mineral (A) on heating in presence of air gives a gas (B). The...
Text Solution
|
- When gas A is passed through dry KOH at low temperature, a deep red co...
Text Solution
|
- The gas liberated on heating a mixture of two salts with NaOH, gives a...
Text Solution
|
- A certain salt (X) gives the following tests : (a) Its aqueous solut...
Text Solution
|
- An unknown inorganic compound (X) loses it water of crystallisation on...
Text Solution
|
- A compound (X) on heating with an excess of NaOH solution gives a gas ...
Text Solution
|