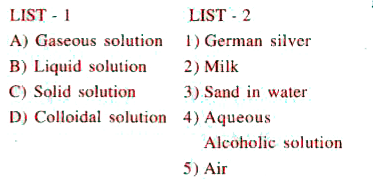A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
AAKASH SERIES|Exercise Objective Exercise -2|110 VideosSOLUTIONS
AAKASH SERIES|Exercise Objective Exercise -3 (PREVIOUS NEEET/AIPMT )|24 VideosSOLUTIONS
AAKASH SERIES|Exercise Subjective Exercise-3 (Problems)|7 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 VideosSTATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE -PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- IV)|2 Videos
AAKASH SERIES-SOLUTIONS -Objective Exercise -1
- Which of the following differ from the others
Text Solution
|
- Occlusion of Hydrogen on Palladium is an example for ………type solution
Text Solution
|
Text Solution
|
- The units of Molarity are
Text Solution
|
- To halve the molarity of a solution the following should be adopted
Text Solution
|
- Molarity of the liquid HCl if density of the solution is 1.17 g/cc is
Text Solution
|
- A student has 100mL of 0.1 M KCl solution. To make it 0.2 M, he has to
Text Solution
|
- Density of 2.05M solution of acetic acid in water is 1.02 g/mL, the mo...
Text Solution
|
- The volume of decamolar aquous solutions of hydrochloric acid is requi...
Text Solution
|
- The units of Normality are
Text Solution
|
- The following is not a fixed quantity
Text Solution
|
- In the reaction 2NaOH+H3PO4 to Na2HPO4 + 2H2O, the Equivalent weight o...
Text Solution
|
- 5 ml of 1N HCl, 20 ml of N/2 H2SO4 and 30 ml of N/3 HNO3 are mixed tog...
Text Solution
|
- The volumes of 4N HCl and 10N HCl required to make 1L of 6 N HCl are
Text Solution
|
- Which of the following acid has the same molecular weight and equivale...
Text Solution
|
- M' is the molecular weight of KMnO4 The equivalent weight of KMnO4 whe...
Text Solution
|
- Molecular weight of KMnO4 is "M". In a reaction KMnO4 is reduced to K2...
Text Solution
|
- The equivalent weight of Hypo in the reaction Na2S2O3 + Cl2 + H2O to N...
Text Solution
|
- Molecular weight of Mohr's salt is 392. Its equivalent weight when it ...
Text Solution
|
- The equivalent weight of CH4 in the reaction CH4+2O2 to CO2 + 2H2O is ...
Text Solution
|