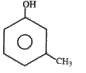
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-3 (More than one correct answer Questions)|9 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-3 (Linked Comprehension type questions)|6 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-2 (Integer answer Type questions)|7 VideosPOLYMERS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4|59 VideosPRINCIPLES OF METALLURGY
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-2 (LONG ANSWER QUESTIONS )|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PRACTICAL CHEMISTRY-PRACTICE SHEET-3 (Single answer questions)
- Compound (X) of molecular formula C(4)H(8) takes up one equivalent of ...
Text Solution
|
- An unknown organic compound is soluble in water. It gives negative tes...
Text Solution
|
- Compound [X] gives positive test with 2, 4-DNP and with I(2) // NaOH c...
Text Solution
|
- Lassaigne's extract of m-nitrochlorobenzene is acidified with dil. HNO...
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- An organic compound [X] of the formula C(7)H(8)O is soluble in NaOH bu...
Text Solution
|