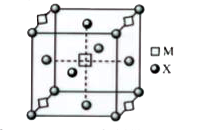
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - I)|31 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - II)|6 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - I (EXERCISE - I)|68 VideosREVISION EXERCISE
AAKASH SERIES|Exercise COMPLEX COMPOUNDS|47 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-LEVEL - I (EXERCISE - II)
- A compound is formed by elements A and B. This crystallizes in the cub...
Text Solution
|
- In a compound atoms of element 'Y' form C.C.P. lattice and those of el...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- The intermetallic compound LiAg crystallizes in cubic lattice in which...
Text Solution
|
- In the crystals of which of the following ionic compounds would you ex...
Text Solution
|
- Gold crystallizes with a
Text Solution
|
- When molten zinc is cooled to solid state, it assumes HCP structure. T...
Text Solution
|
- Sodium crystallizes in a bcc lattice, hence the coordination number of...
Text Solution
|
- The metal having 26% void space in its crystal structure is
Text Solution
|
- The percentage of void space of a metallic element crystallising in a ...
Text Solution
|
- A metal crystallises in a simple cubic unit cell of edge length 6.22 Å...
Text Solution
|
- Sodium metal crystallises in a body-centred cubic lattice with the cel...
Text Solution
|
- Copper crystallises in a f.c.c. lattice, the length of the unit cell i...
Text Solution
|
- The radius of an atom of an element is 80 pm. If it crystallises as a ...
Text Solution
|
- A metal has bcc structure and the edge length of its unit cell is 3.04...
Text Solution
|
- If the radius of K^(+) and F^(-) are 133 pm and 136 pm respectively, t...
Text Solution
|
- Schottky defect causes
Text Solution
|
- What type of crystal defect is indicated in the diagram below |{:(Na...
Text Solution
|
- Schottky - Wagner defects are mostly found in
Text Solution
|
- Which among the following is likely to have Schottky defect.
Text Solution
|