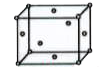
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - II)|6 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - III)|5 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - I (EXERCISE - II)|77 VideosREVISION EXERCISE
AAKASH SERIES|Exercise COMPLEX COMPOUNDS|47 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-LEVEL - II LECTURE SHEET (EXERCISE - I)
- For a solid with the following structure, the coordination number of t...
Text Solution
|
- For the structure given below the site marked as S is a :
Text Solution
|
- A solid A^(+)B^(-) has the B^(-) ions arranged as below. If the A^(+) ...
Text Solution
|
- In FCC crystal, which of the following shaded planes contains the foll...
Text Solution
|
- In the closest packing of atom B, the radius of atom A that can be fit...
Text Solution
|
- Distance between tetrahedral void and octahedral void in the lattice w...
Text Solution
|
- The two types of holes which occur in any close-packed structures are
Text Solution
|
- In face centered cubic structure, the octahedral voids are located at ...
Text Solution
|
- A non-stoichiometric compound Cu(1.8)S is formed due to incorporation ...
Text Solution
|
- Consider following statements I: If three Fe^(2+) are missing from i...
Text Solution
|
- The n-type semiconductor is obtained when Si is doped with
Text Solution
|
- Superconductors are technologically and commercially important substan...
Text Solution
|
- When ferromagnetic substances are heated strongly, its magnetic moment
Text Solution
|
- Silica (SiO(2)) can be crystalline as well as amorphous, with followin...
Text Solution
|
- In a face centred cubic arrangement of A and B atoms whose A atoms are...
Text Solution
|
- Which of the following statements are false:
Text Solution
|
- Edge length of NaCl unit cell is 552 pm. Then which are correct statem...
Text Solution
|
- Which is true
Text Solution
|
- A metallic element crystallises into a lattice containing a sequence o...
Text Solution
|
- In an FCC unit cell atoms are numbered as shown below the atoms not to...
Text Solution
|