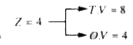
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 2|30 VideosSOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 3|30 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - IV)|4 VideosREVISION EXERCISE
AAKASH SERIES|Exercise COMPLEX COMPOUNDS|47 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-PRACTICE SHEET - 1
- Which of the following are not the characteristics of crystalline soli...
Text Solution
|
- Glasses and plastics are
Text Solution
|
- If the three interaxial angles defining the unit cell are all equal in...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statment(s) is (are) correct?
Text Solution
|
- In the unit cell of NaCl, which of the following statements are correc...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- In a close packed lattice containing 'n' particles, the number of tetr...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Following is the fact about the newly discovered superconductor of C(6...
Text Solution
|
- Following is the fact about the newly discovered superconductor of C(6...
Text Solution
|
- The ionic radii of Na^(+), K^(+) and Rb^(+) are 97, 133 and 147 pm, re...
Text Solution
|
- Match the nature of bonding mentioned in list I with the solids in lis...
Text Solution
|
- Match the elements (in Column - I) with the shape of the crystal (in C...
Text Solution
|
- The number of atoms in HCP unit cell is
Text Solution
|
- Number of atoms per unit cell for body centered cubic system is .........
Text Solution
|
- A substance A(x)B(y) crystallises in a face centered cubic lattice. At...
Text Solution
|
- If 3 moles of atoms are present in the packing of pattern ABC ABC ABC....
Text Solution
|
- A spinel is an important class of oxides consisting of two types of me...
Text Solution
|
- The number of unit cells present in a cubic shaped ideal crystal of Na...
Text Solution
|