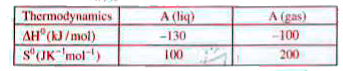A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|7 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)|2 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - I (MAIN))|13 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Questions For Descriptive Answers|28 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) STRAIGHT OBJECTIVE TYPE QUESTIONS)
- When 3.0 mole of an ideal diatomic gas is heated and compressed simult...
Text Solution
|
- underset(P(1), V(1), T(1))("State") A overset("Rev")rarr underset(P(2)...
Text Solution
|
- During winters, moisture condenses in the form of dew and can be seen ...
Text Solution
|
- Assuming Delta H^(0) and Delta S^(0) do not change with temperature th...
Text Solution
|
- For the reaction X(2)O(4(l)) rarr 2XO(2(g)), Delta U= 2.1 "kcal", Delt...
Text Solution
|
- The correct signs of Delta S for the following four processes respecti...
Text Solution
|
- Work (W) is the path function. Which of the following order is incorre...
Text Solution
|
- Standar entropies of x(2), y(2) and xy(3) are 60,40 and 50JK^(-1) mol^...
Text Solution
|