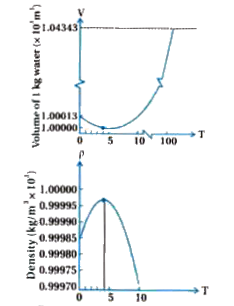
Text Solution
Verified by Experts
Topper's Solved these Questions
THERMAL PROPERTIES OF MATTER
KUMAR PRAKASHAN|Exercise Try Yourself (VSQs)|84 VideosTHERMAL PROPERTIES OF MATTER
KUMAR PRAKASHAN|Exercise Information : Higher Order Thinking Skills (HOTS)|8 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
KUMAR PRAKASHAN|Exercise SECTION-F (SECTION-D) QUESTIONS PAPER|1 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Question Paper|11 Videos
Similar Questions
Explore conceptually related problems