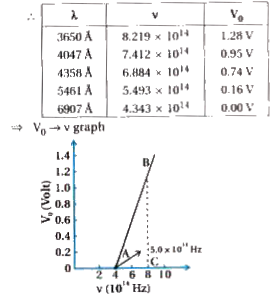
Text Solution
Verified by Experts
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
KUMAR PRAKASHAN|Exercise Section-B Numerical from textual Exercise(Numerical From .DARPAN. based on textbook)|11 VideosDUAL NATURE OF RADIATION AND MATTER
KUMAR PRAKASHAN|Exercise Section-B (QUESTIONS)|7 VideosDUAL NATURE OF RADIATION AND MATTER
KUMAR PRAKASHAN|Exercise Section-B Numerical from textual Exercise|19 VideosCURRENT ELECTRICITY
KUMAR PRAKASHAN|Exercise SECTION [D] MULTIPLE CHOICE QUESTIONS (MCQs) (MCQs ASKED IN BOARD EXAM AND GUJCET)|23 VideosELECTRIC CHARGES AND FIELDS
KUMAR PRAKASHAN|Exercise SECTION D MCQS ASKED IN COMPETITIVE EXAMES (MCQS AKSED IN BOARD EXAM AND GUJCET)|14 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-DUAL NATURE OF RADIATION AND MATTER-Section-B Numerical from textual Exercise(Additional Exercise)
- (a) Estimate the speed with which electrons emitted from a heated emit...
Text Solution
|
- (a) A monoenergetic electron beam with electron speed of 5.20 xx 10^(6...
Text Solution
|
- An electron gun with its collector at a potential of 100 v fires out e...
Text Solution
|
- (a) An X-ray tube produces a continuous spectrum of radiation with its...
Text Solution
|
- In an accelerator experiment on high-energy collisions of electrons wi...
Text Solution
|
- Estimating the following two numbers should be interesting. The first ...
Text Solution
|
- Ultraviolet light of wavelength 2271 Å from a 100 W mercury source irr...
Text Solution
|
- Monochromatic radiation of wavelength "640.2 nm "(1nm = 10^(-9) m) fro...
Text Solution
|
- A mercury lamp is a convenient source for studying frequency dependenc...
Text Solution
|
- The work function for the following metals is given: Na: 2.75 eV, K: 2...
Text Solution
|
- Light of intensity 10^(-5) W m^(-2) falls on a sodium photo-cell of su...
Text Solution
|
- Crystal diffraction experiments can be performed using X-rays, or elec...
Text Solution
|
- (a) Obtain the de Broglie wavelength of a neutron of kinetic energy 15...
Text Solution
|
- An electron microscope uses electrons accelerated by a voltage of 50 k...
Text Solution
|
- The wavelength of a probe is roughly a measure of the size of a struct...
Text Solution
|
- Find the typical de Broglie wavelength associated with a He atom in he...
Text Solution
|
- Compute the typical de Broglie wavelength of an electron in a metal at...
Text Solution
|
- Answer the following questions: (d) Every metal has a definite work ...
Text Solution
|