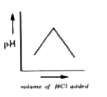
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE -IV LEVEL -II (ADVANCED) (Straight Objective Type Questions))|12 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE -IV LEVEL -II (ADVANCED) (More than One correct answer Type Questions))|14 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE -III LEVEL -II (ADVANCED ) (Integer Type Questions))|16 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|20 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-IONIC EQUILIBRIUM -LECTURE SHEET (EXERCISE -IV LEVEL -I (MAIN ) (Straight Objective Type Questions))
- Given that the data for neutralization of a weak acid (HA) and strong ...
Text Solution
|
- Titration curve if a strong base is titrated with srtrong acid is:
Text Solution
|
- At a certain temperature ,the solubility of the salt Mm An in water i...
Text Solution
|
- The correct representation for solubility product of SnS(2) , is
Text Solution
|
- For the electrolyte of type ,A2B, K(sp) is given. Then its solubility...
Text Solution
|
- The solubilty product of the electrolyte of the type A2B3 is (s is the...
Text Solution
|
- K(sp) of a salt , having the general formula MX2 in water 4xx10^(-1...
Text Solution
|
- Determine the mass by mass percentage concentration of a 100 g salt so...
Text Solution
|
- Let the solubility of an aqueous solution of Mg(OH)(2), be "X^(@) then...
Text Solution
|
- The solubility product of a rare earth metal hydroxide M(OH)3 at ro...
Text Solution
|
- The volume of water needed to dissolve 1g of BaSO4 (K(sp) =1.1 xx 10^...
Text Solution
|
- Which of the following is wrong ?
Text Solution
|
- Assertion (A) : P^(H) of an aqueous solution of acetic acid remians...
Text Solution
|
- Which pair will show common ion effect ?
Text Solution
|
- The addition of NaCl to AgCI decreases the solubility of AgCl, because
Text Solution
|
- In which of the following, the solubility of AgCl will be maximum?
Text Solution
|
- The solubility of PbSO4 "in" 0.01 M Na2SO4 solution is (K(sp) of Pb...
Text Solution
|
- 100 mL , each of 0.25 M NaF and 0.01 5 Ba (NO)3 are mixed K(sp) of Ba...
Text Solution
|
- The molar solubility of Pbl(2) in 0.2M Pb(NO(3))(2) solution in terms ...
Text Solution
|
- At 298 K , the K(sp) value of Fe(OH)3 in aqueous solution is 3.8 xx ...
Text Solution
|