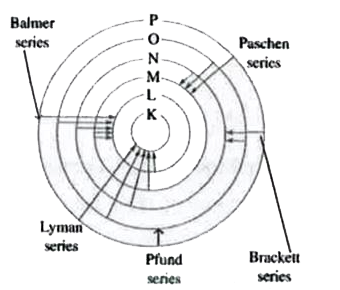
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - II (Type-I) (Practice sheet - 1 (Section -C : Matching/Straight objective type questions))|2 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - II (Type-I) (Lecture Sheet - 2 (Section-A : More than one correct answer type questions))|5 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - II (Type-I) (Practice sheet - 1 (Section-A : More than one correct answer type questions))|5 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
AAKASH SERIES-ATOMIC STRUCTURE-Level - II (Type-I) (Practice sheet - 1 (Section - B : Linked comprehension Type questions))
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|