Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PERIODIC TABLE-QUESTIONS FOR DESCRIPTIVE ANSWERS
- What are anhydrides? Write exmples of acidic and basic anhydrides.
Text Solution
|
- Alkali metals are good reductants and halogens are good oxidants. Why?
Text Solution
|
- Comment on the following : (a) vertical relationship of elements, (b...
Text Solution
|
- How many periods are needed to accommodate all elements up to Z value ...
Text Solution
|
- How is polarisation power useful in explaining the diagonal relationsh...
Text Solution
|
- I1 and I2 of an element are 700 and 1200 kJmol""^- If 1000 kJ "mol"^(-...
Text Solution
|
- The energy required for the following process is 1.96 xx 104" kJ mol"^...
Text Solution
|
- Compare the ionisation potentials of He and He^(+). Account for the di...
Text Solution
|
- Bond energies of H2, F2 and HF are respec tively 104.2, 36.6 and 134.6...
Text Solution
|
- Ionisation enthalpy of chlorine is "13 eV atom"^(-1) Electron gain ent...
Text Solution
|
- Be forms Be^(2+) and Al^(3+) forms Al^(3+). If the radii of Be^(2+) an...
Text Solution
|
- Among F((g))^(-),Cl((g))^(-0),Br((g))^(-) and I((g))^(-), which one r...
Text Solution
|
- Among Li(g), Cs(g), Cl(g) and Cl((g))^(-) which one requires the leas...
Text Solution
|
- If we assumed that each orbital can accomidate three electrons, then c...
Text Solution
|
- An element with the electronic configuration [Xe] 6s^(2)4f^(14)5d^(1) ...
Text Solution
|
- Calculate the IP(3) of lithium.
Text Solution
|
- H-H and Cl-Cl bond length are 0.74Å and 1.98Å What is the approximate ...
Text Solution
|
- X and Y are two univalent elements. In order to form electrovalent com...
Text Solution
|
- Few transition elements show +1 oxidation state. Explain.
Text Solution
|
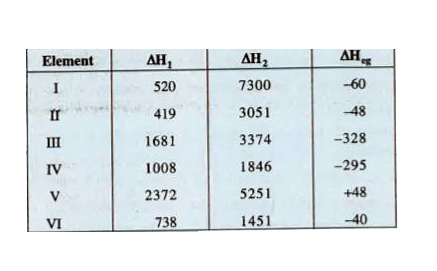
- The first (DletaH) and second ionisation enthalipies (DeltaH(2)) in kJ...
Text Solution
|