Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
VIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 (Single or more than one option questions)|16 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 (Linked Comprehension type questions)|6 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 (Match the following questions)|2 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VIA GROUP ELEMENTS-PRACTICE SHEET - 3 (Integer answer type Questions)
- How many S-S bond are present in SO(3) trimer
Text Solution
|
- How many S-S bond are there in S(2)O(7)^(-2)
Text Solution
|
- How many S-S bond in polythionic acid having molecular formula H(2)S(5...
Text Solution
|
- The total no. of diprotic acids among the following is H(3)PO(4), H(2)...
Text Solution
|
- Number of hydorxyl groups present in pyrosulphuric acid is
Text Solution
|
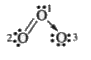
- The formal charges on the three oxygen atoms in O(3) molecule are
Text Solution
|