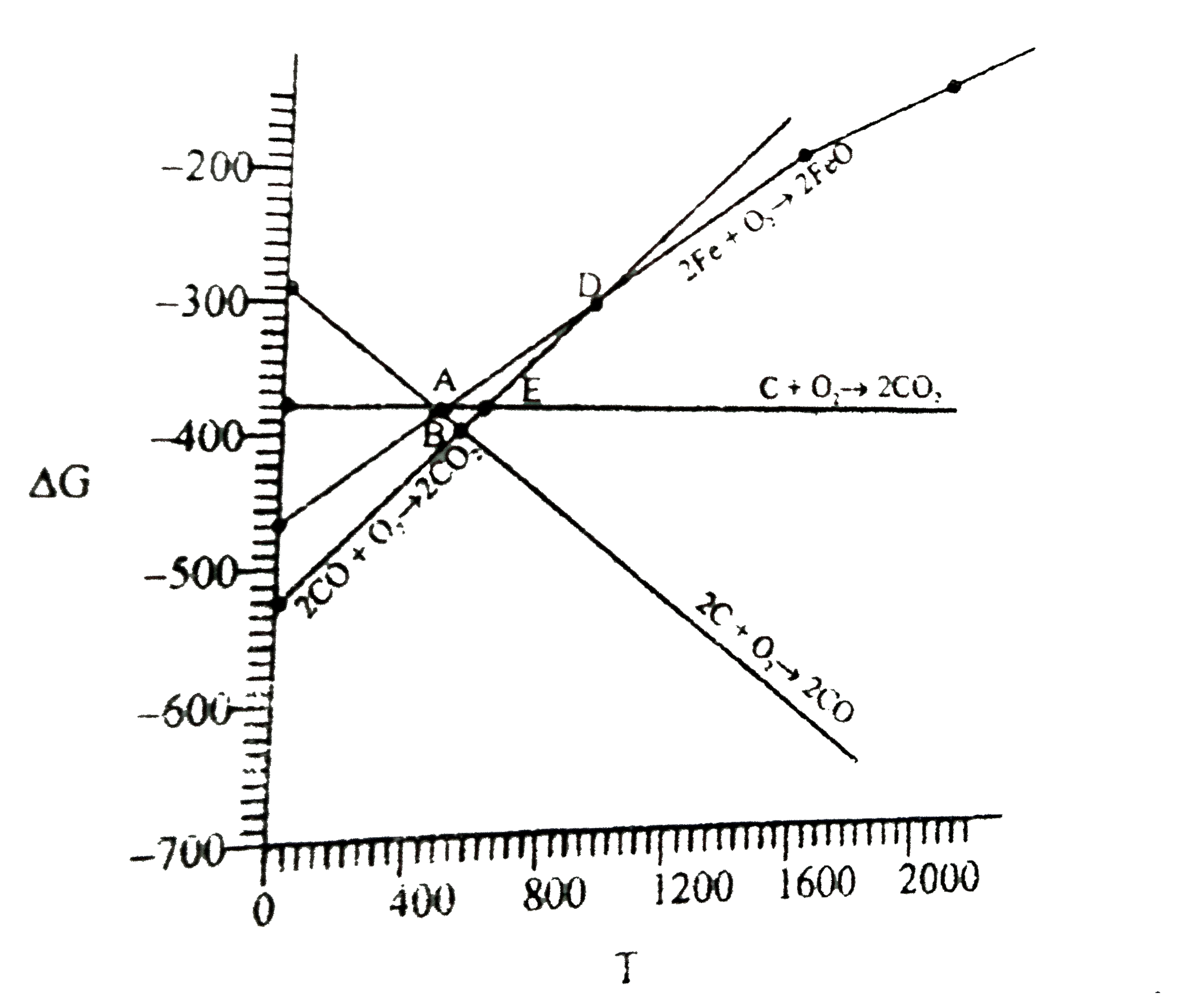
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLE AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Short answer type Question|18 VideosGENERAL PRINCIPLE AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Matching The columns|5 VideosELECTROCHEMISTRY
NCERT EXEMPLAR ENGLISH|Exercise All Questions|67 VideosHALOALKANES AND HALOARENES
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|3 Videos
Similar Questions
Explore conceptually related problems