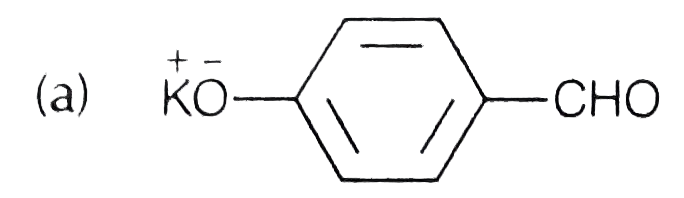
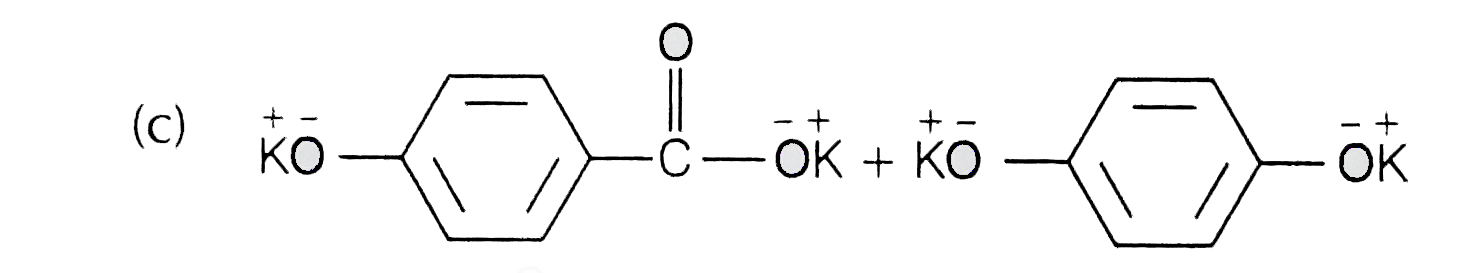
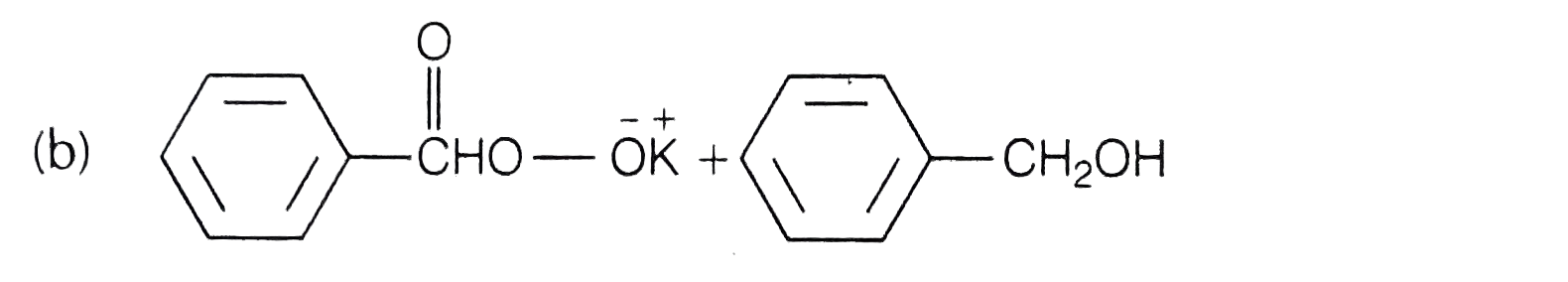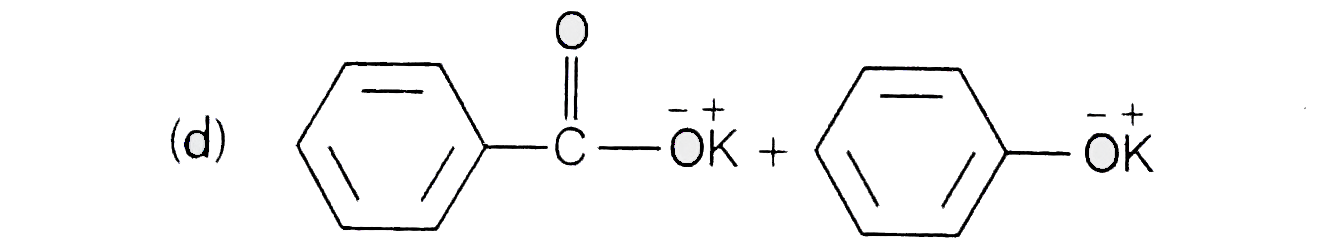A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDE, KETONES AND CARBOXYLIC ACIDS
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type Questions|28 VideosALDEHYDE, KETONES AND CARBOXYLIC ACIDS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|4 VideosALCOHOLS, PHENOLS AND ETHERS
NCERT EXEMPLAR ENGLISH|Exercise All Questions|74 VideosAMINES
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER|3 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-ALDEHYDE, KETONES AND CARBOXYLIC ACIDS-Long Answer Type Questions
- Which product is formed when the compound is treated with concentrated...
Text Solution
|
- An alkene 'A' (molecular formula C(5)H(10)) on ozonolysis gives a mixt...
Text Solution
|
- An aromatic compound 'A' (Molecular formula C(8)H(8)O)) gives positive...
Text Solution
|
- Write down functional isomers of a carbonlyl compound with molecular f...
Text Solution
|
- When liquid 'A' is treated with a freshly prepared ammoniacal silver n...
Text Solution
|