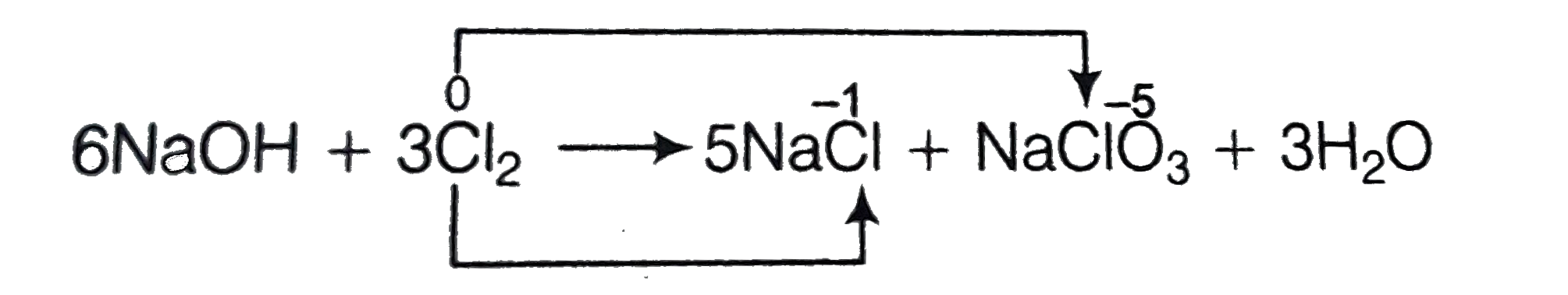A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Matching The Columns|5 VideosP-BLOCK ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Assertion and Reason|6 VideosHALOALKANES AND HALOARENES
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|3 VideosPOLYMER
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Question|5 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-P-BLOCK ELEMENTS-Long Answer Type Questions
- If chlorine gas is passed through hot NaOH solution, two changes are o...
Text Solution
|
- An amorphous soild A burns in air to form a gas B which turns lime w...
Text Solution
|
- On heating lead (II) nitrate gives a brown gas " A". The gas " A" on ...
Text Solution
|
- On heating compound (A) gives a gas (B) which is a constituent of air....
Text Solution
|