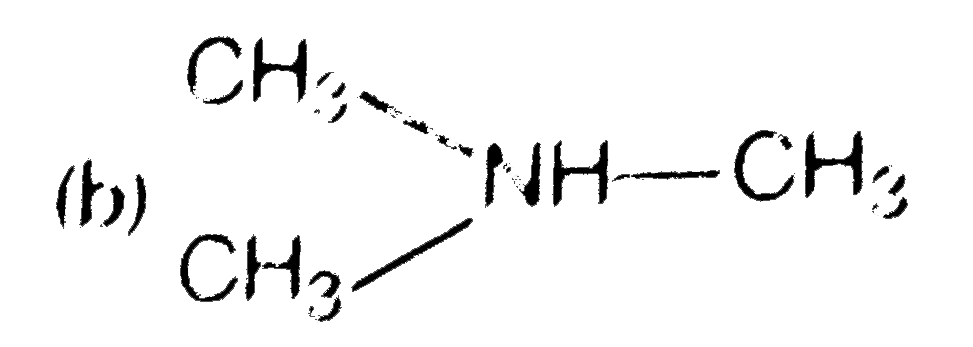
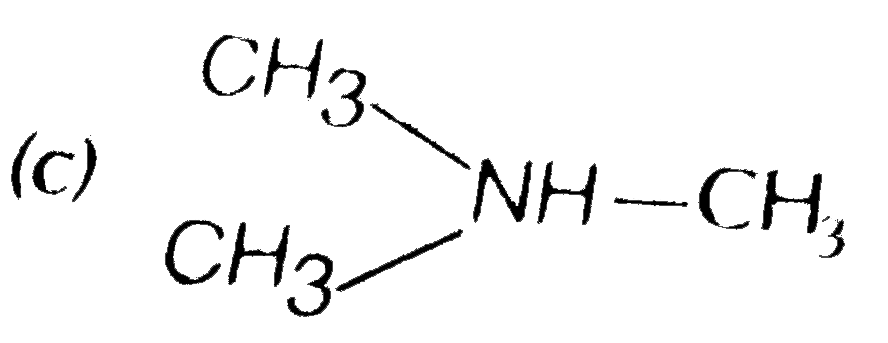
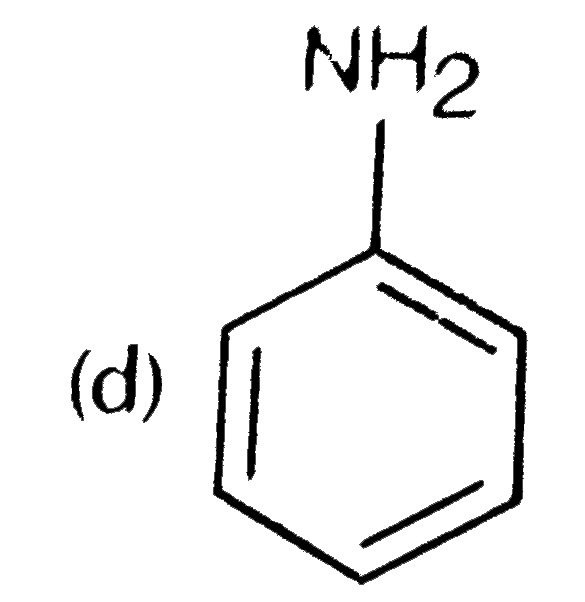
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-AMINES-LONG ANSWER
- The most reactive amine towards dilute hydrochloric acid is…
Text Solution
|
- A hydrocarbon 'A' (C(4)H(8)) on reaction HCl gives a compound 'B', (C(...
Text Solution
|
- A colours substance 'A' (C(6)H(7)) is sparingly soluble in water and g...
Text Solution
|
- Predict the reagent or the product in the following reaction sequence.
Text Solution
|