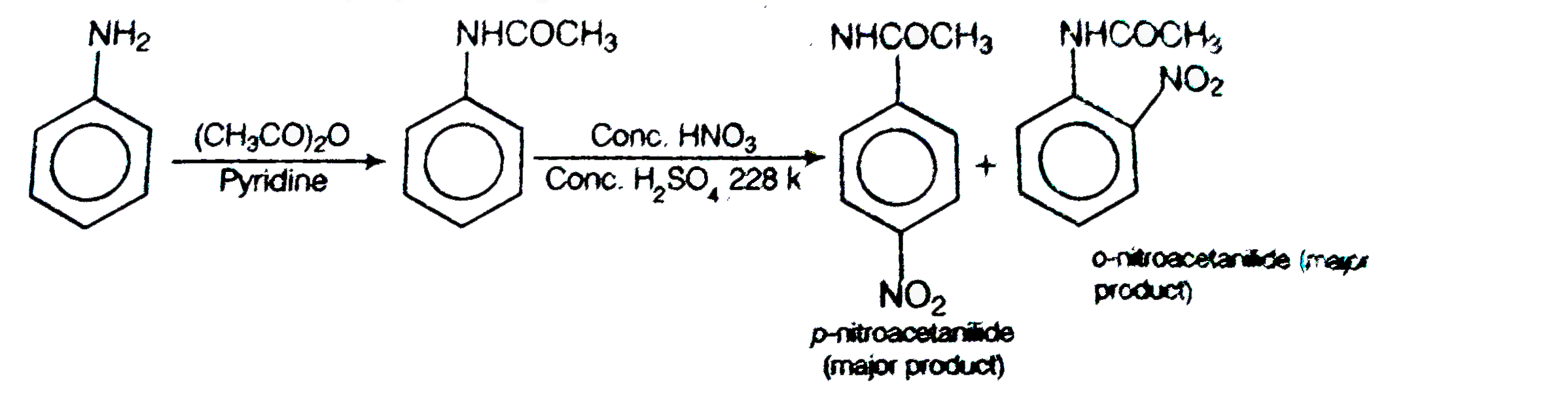
Text Solution
Verified by Experts
Topper's Solved these Questions
AMINES
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER|3 VideosAMINES
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER|3 VideosALDEHYDE, KETONES AND CARBOXYLIC ACIDS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|4 VideosBIOMOLECULES
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|5 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-AMINES-SATQ
- What is the role of HNO(3) in the nitrating mixture used for nitration...
Text Solution
|
- Why is NH(2) group of aniline acetylated before carrying our nitration
Text Solution
|
- What is the product when C(6)H(5)CH(2)NH(2) reacts with HNO(3)?
Text Solution
|
- What is the best reagent to convert nitrile to primary amine
Text Solution
|
- Give the structure of 'A' in the following reaction.
Text Solution
|
- What is Hinsberg reagent?
Text Solution
|
- Why is benzene diazonium chloride not stored and is used immediately a...
Text Solution
|
- Why does acylation of -NH(2) of aniline reduces its activating effect?
Text Solution
|
- Explain why MeNH(2) is stronger base than MeOH?
Text Solution
|
- What is the role of pyridine in the acelation reaction of amines?
Text Solution
|
- Under the reaction condition (acidic, basic) the coupling reaction of ...
Text Solution
|
- Predict the product of reaciton for aniline with bromine in non-polar...
Text Solution
|
- Arraange the following compounds in increasing order of dipole moment?...
Text Solution
|
- What is the structure and IUPAC name of the compound, allyl amine?
Text Solution
|
- Write down the IUPAC name of
Text Solution
|
- A compound Z with molecular formula C(3)H(9)N reacts with C(6)H(5)SO(2...
Text Solution
|
- A primary amine, RNH(2) can be reacted with CH(3)-X to get secondary a...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Why is aniline soluble in aqueous HCl?
Text Solution
|
- Suggest a route by which of the following conversion can be accomplish...
Text Solution
|