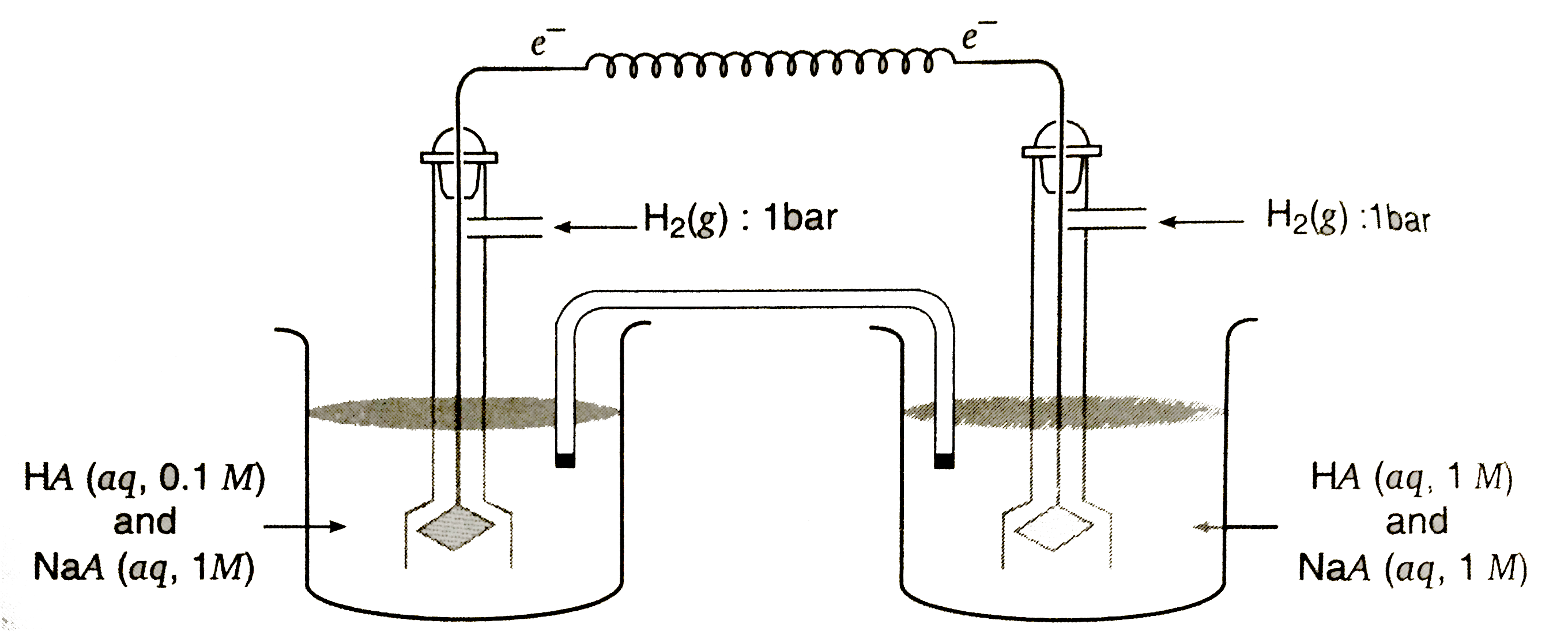
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise LEVEL-3|37 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Match the column|6 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|14 VideosDILUTE SOLUTION
NARENDRA AWASTHI ENGLISH|Exercise leval-03|23 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|15 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-ELECTROCHEMISTRY-LEVEL-2
- For a cell reaction 2H2(g)+O2(g)to2H2O(l)/\rS(198)^(@)=-0.32KJ//k . Wh...
Text Solution
|
- What is the potential of an electrode which originally contained 0.1 M...
Text Solution
|
- The standard reduction potential of normal calomel electrode and reduc...
Text Solution
|
- Determine the potential of the following cell: Pt|H2(g,0.1 "bar")|H^...
Text Solution
|
- Copper reduced NO(3)^(-) into NO and NO(2) depending upon conc.of HNO(...
Text Solution
|
- For the cell, Pt|Cl2(g,0.4"bar")|Cl^(-)(aq,0.1M)"||"Cl^(-)(aq),0.01M)|...
Text Solution
|
- The chlorate ion can disproportinate in basic solution according to re...
Text Solution
|
- A cell diagram shown below contains of one litre of buffer solution of...
Text Solution
|
- Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq,0.01M)|H2(g,1"bar")|Pt(s) w...
Text Solution
|
- calculate the e.m.f (in V) of the cell: Pt|H2(g)|BOH(Aq)"||"HA(Aq)|H...
Text Solution
|
- Calculate the potential of a half cell having reaction :Ag2S(s)+2e^(-)...
Text Solution
|
- The conductivity of 0.1 N NaOH solution is 0.022 Scm^(-1).When equal v...
Text Solution
|
- In above question after formation of NaCl, further 0.1 N HCl is added,...
Text Solution
|
- Given the following molar conductivity at 25^(@) C:, HCl,426Omega^(-1)...
Text Solution
|
- Equivalent conductivity of BaCl2,H2SO4 and HCl, are x1,x2"and"x3Scm^(-...
Text Solution
|
- The conductivity of 0.001M Na2SO4 solution is 2.6xx10^(-4)Scm^(-1) and...
Text Solution
|
- The dissociation constant of a weak acid is 1.6xx10^(-5) and the molar...
Text Solution
|
- Three electrolytic cells X,Y,Z containing solution of NaCl, AgNO3 and ...
Text Solution
|
- During electrolysis of H2SO4(aq) with high charge density, H2S2O8 form...
Text Solution
|
- Zn(s)|Zn(CN)4^(2-)(0.5M),CN^(-)(0.01)"||"Cu(NH3)4^(2+)(0.5M),NH3(1M)|C...
Text Solution
|