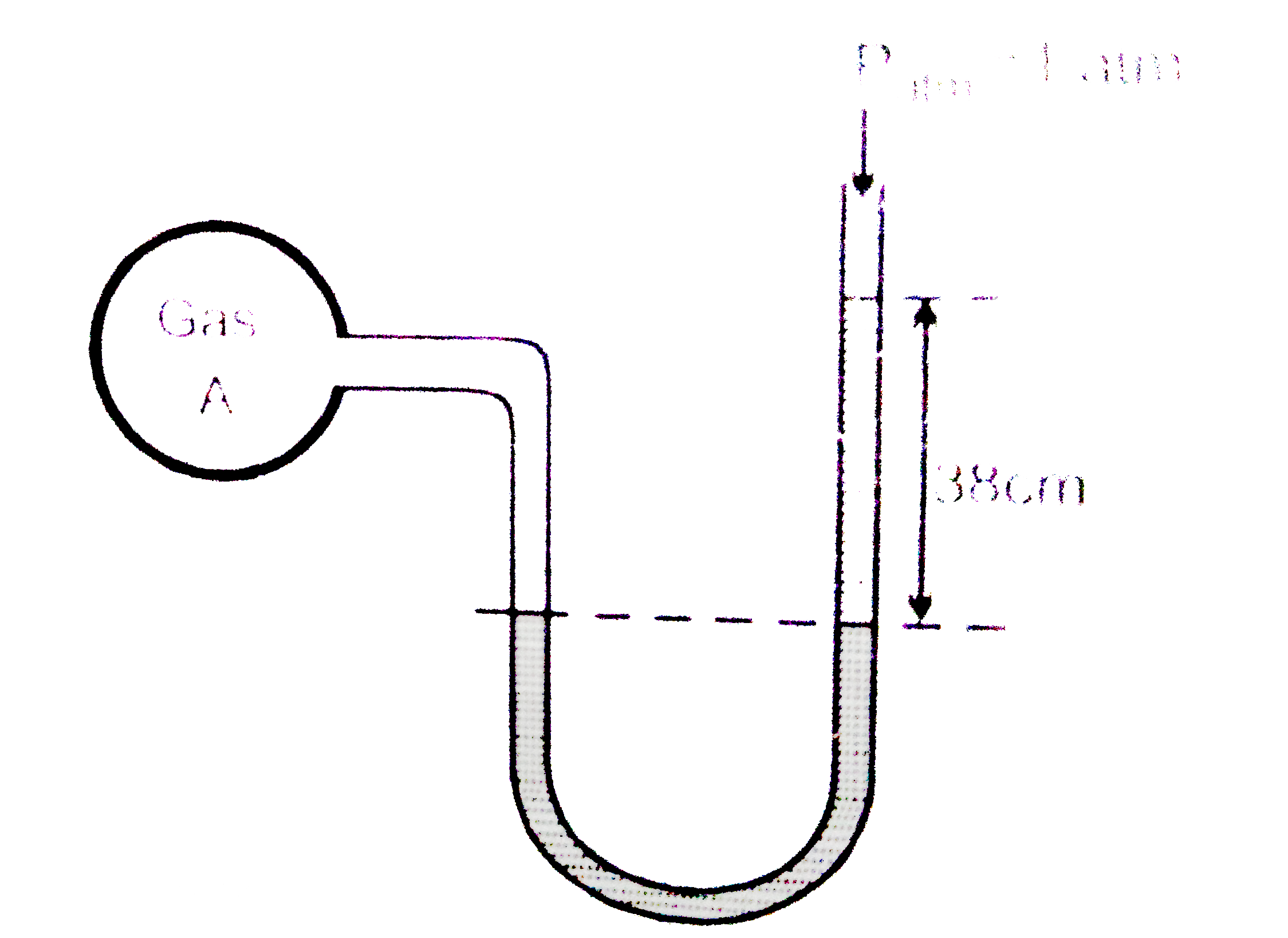A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise One or More Answers is/are Correct|20 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Match the Column|7 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Level 3 Passage 2|6 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|14 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-GASEOUS STATE-Level 3 Passage 3
- A monometer contains a liquid of density 5.44g//cm^(3) is attached to ...
Text Solution
|
- A manometer contains a liquid of density 5.44g//cm^(3) is attached to ...
Text Solution
|
- A monometer contains a liquid of density 5.44g//cm^(3) is attached to ...
Text Solution
|
- A monometer contains a liquid of density 5.44g//cm^(3) is attached to ...
Text Solution
|