Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise-3.1.1|5 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise-3.1.2|24 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Conversions (Name Reaction and reagents :)|24 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise ETHERS (OBJECTIVE EXERCISE-4)|50 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALDEHYDES AND KETONES-Problem
- Arrange the following compounds in increasing order of their reactivit...
Text Solution
|
- Compound A, C5H(10)O, forms a phenyl hyrazone, given negative Tollen's...
Text Solution
|
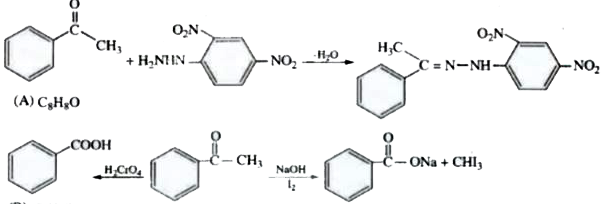
- An organic compound (A) with molecular formula C(8)H(8)O forms an oran...
Text Solution
|
- An alkene , C6H(12) after ozonolysis yielded two products. One of thes...
Text Solution
|
- Two organic compounds (A) and (B) with molecular formula, C3H6O , reac...
Text Solution
|
- Although aldehydes are easily oxidisable, propanal can conveniently be...
Text Solution
|
- Aldehydes do not form stable hydrates, yet chloral hydrate is readily ...
Text Solution
|
- How is acetaldehyde distinguished from acetone?
Text Solution
|
- What are metaldehyde and paraldehyde?
Text Solution
|
- What products are obtained in a crossed Cannizzaro reaction, involving...
Text Solution
|
- An organic compound having molecular formula C5H(10) O exists in two c...
Text Solution
|
- Benzaldehyde is treated with acetaldehyde in presence of dilute alkali...
Text Solution
|
- How do you distingush between benzaldehyde and acetophenone?
Text Solution
|
- Given the structures of the compounds formed when the reaction takes p...
Text Solution
|
- C(6)H(6) + C(2)H(5)Cl overset(AlCl(3))rarr X underset((CH(3)COO)(2)Mn)...
Text Solution
|
- How do you prepare acetophenone from benzene?
Text Solution
|
- What is the rate determining step in Cannizzaro's reaction?
Text Solution
|
- Predict products in the following reactions and write their structures...
Text Solution
|
- Can (CH3)3 CCHO undergo Cannizzaro reaction ?
Text Solution
|
- C(6)H(6) + C(2)H(5)Cl overset(AlCl(3))rarr X underset((CH(3)COO)(2)Mn)...
Text Solution
|