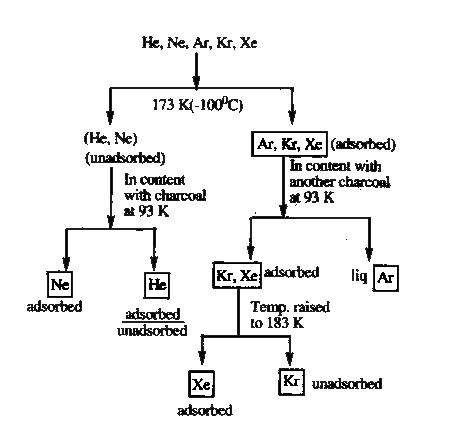
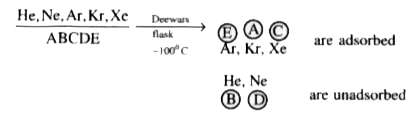A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NOBLE GASES
AAKASH SERIES|Exercise LEVEL - II (LECTURE SHEET) (EXERCISE - III MATCH THE FOLLOWING QUESTIONS)|5 VideosNOBLE GASES
AAKASH SERIES|Exercise LEVEL - II (LECTURE SHEET) (EXERCISE - IV INTEGER ANSWER TYPE QUESTIONS)|10 VideosNOBLE GASES
AAKASH SERIES|Exercise LEVEL - II (LECTURE SHEET) (EXERCISE - I SIGGLE & ONE OR MORE THAN ONE CORRECT ANSWERS)|12 VideosNITROGEN CONTAINING COMPOUNDS
AAKASH SERIES|Exercise Conversions|18 VideosNUCLEAR CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Integer answer type Questions )|8 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-NOBLE GASES-LEVEL - II (LECTURE SHEET) (EXERCISE - II LINKED COMPREHENSION TYPE QUESTIONS)
- Noble gases A, B, C, D, E are passed through Dewar's flask at-100^@C A...
Text Solution
|
- Noble gases A, B, C, D, E are passed through Dewar's flask at-100^@C A...
Text Solution
|
- Xenon Fluorides : Xenon reacts directly with fluorine on heating at 67...
Text Solution
|
- Xenon Fluorides : Xenon reacts directly with fluorine on heating at 67...
Text Solution
|