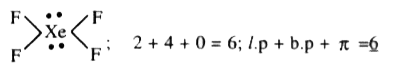Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NOBLE GASES
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Single more than one option questions)|16 VideosNOBLE GASES
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Linked Comprehension type questions )|6 VideosNOBLE GASES
AAKASH SERIES|Exercise LEVEL - II (LECTURE SHEET) (EXERCISE - III MATCH THE FOLLOWING QUESTIONS)|5 VideosNITROGEN CONTAINING COMPOUNDS
AAKASH SERIES|Exercise Conversions|18 VideosNUCLEAR CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Integer answer type Questions )|8 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-NOBLE GASES-LEVEL - II (LECTURE SHEET) (EXERCISE - IV INTEGER ANSWER TYPE QUESTIONS)
- The percentage abundance of Neon gas in air by volume is 1.8 xx 10^(-...
Text Solution
|
- Describe the Fischer - Ringe's method.
Text Solution
|
- Neon is mainly used in discharge tubes at a pressure of mm
Text Solution
|
- XeF6 + x H2O to XeO3 + y HF, the y/x =
Text Solution
|
- Xe + 2PtF6 overset(25^@C)to [XeF]^(+) [ PtF6]^(-) + PtF5 then in the c...
Text Solution
|
- In XeF4 molecule, the no. of lone pairs are x, the air x, the no. of s...
Text Solution
|
- Number of following compounds in which central atom has "+6" oxidation...
Text Solution
|
- Number of following compounds in which central atom has "+6" oxidation...
Text Solution
|
- Number of following compounds in which central atom has "+6" oxidation...
Text Solution
|
- In the Ramsay - Rayleigh's second method, 2NO2 + 2NaOH to NaNO2 + X + ...
Text Solution
|