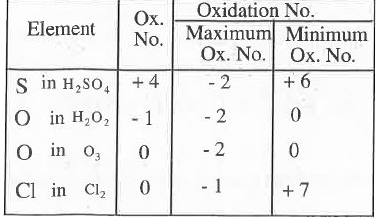
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(Matrix Matching Type Questions)|1 VideosREDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(Integer Type Questions)|5 VideosREDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(More than One correct answer Type Questions)|11 VideosPURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATURE
AAKASH SERIES|Exercise ADDITIONAL PRCATICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|2 VideosREVISION EXERCISE
AAKASH SERIES|Exercise CHEMICAL EQUILIBRIUM|107 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-REDOX REACTIONS-PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(Linked Comprehension Type Questions)
- The oxidation number of an element in a compound decides its nature to...
Text Solution
|
- The oxidation number of an element in a compound decides its nature to...
Text Solution
|
- The oxidation number of an element in a compound decides its nature to...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|