Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise EXERCISE - 4.1.1|5 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise EXERCISE - 4.1.2|13 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-4 (Integer Type Questions)|9 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOXYLIC ACIDS AND DERIVATIVES-CONVERSIONS
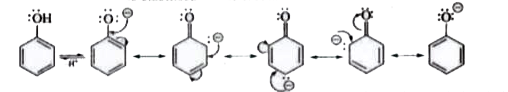
- Explain why carboxylic acids are stronger acids than phenols?
Text Solution
|
- Methanol to acetic acid
Text Solution
|
- Ethanoic acid to propanoic acid
Text Solution
|
- Benzene to methylbenzoate
Text Solution
|
- Propanoic acid to acetic acid
Text Solution
|
- Nitrobenzene to benzoic acid
Text Solution
|
- Benzene to phenylacetic acid .
Text Solution
|
- Benzene to p-nitrobenzoic acid
Text Solution
|
- Benzene to m-nitrobenzoic acid .
Text Solution
|
- Benzene to m-nitroacetophenone .
Text Solution
|
- Ethylbenzene to benzoic acid
Text Solution
|
- Bromobenzene to benzoic acid .
Text Solution
|
- Styrene to benzoic acid
Text Solution
|
- Acetophenone to benzoic acid
Text Solution
|
- Write chemical reactions of affect the 4-Methylacetophenone to benzene...
Text Solution
|
- Butanol-1 to butanoic acid .
Text Solution
|
- Write chemical reactions of affect the Cyclohexene to hexane-1, 6-dioi...
Text Solution
|
- Write chemical reactions of affect the Butanal to butanoic acid transf...
Text Solution
|
- 3-Nitrobromobenzene to 3-nitrobenzoic acid .
Text Solution
|
- Write chemical reactions to affect the following transformations : ...
Text Solution
|