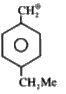
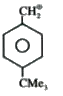
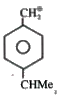A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) Straight ObjectiveType Questions)|21 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) Linked Comprehension Type Questions)|3 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise PRACTICE SHEET - EXERCISE-III (LEVEL - II Integer Type Questions)|8 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-ADDITIONAL PRACTICE EXERCISE (LEVEL - I (MAIN) Straight Objective Type Questions)
- Which order is correct regarding stability of intermediates. (I) CH(...
Text Solution
|
- Select the most stable intermediate.
Text Solution
|
- These are three canonical structures of napthalene. Examine them and f...
Text Solution
|
- CH(2)=underset((I))(CH)-CH=CH-CH(3) is more stable than CH(3)-underset...
Text Solution
|
- In which of the following molecules pi- electron density in ring is ma...
Text Solution
|
- In which of the following molecules pi- electron density in ring is mi...
Text Solution
|
- Which of the following compound is non aromatic?
Text Solution
|
- Among the following molecules, the correct order of C - C bond length ...
Text Solution
|
- C(1)-C(2) bond is shortest in
Text Solution
|
- Which of the following has the longest C - O bond ?
Text Solution
|
- Among these compounds, the correct order of C - N bond lengths is
Text Solution
|
- {:("(I) "CH(3)O-CH=CH-NO(2), "(II) "CH(2)=CH-NO(2)), ("(III) "CH(2)=CH...
Text Solution
|
- Select the least acidic compound.
Text Solution
|
- Write correct order of acidic strength of following compounds
Text Solution
|
- Arrange the given phenols in their decreasing order of acidity S...
Text Solution
|
- Which one of the following is the most acidic?
Text Solution
|
- Which one of the following phenols will show the highest acidity?
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- The correct pKa order of the following acids is
Text Solution
|
- Which of the following compound is having the highest P^(ka) value
Text Solution
|