A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II (STRAIGHT OBJECTIVE TYPE QUESTIONS))|10 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS))|5 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - I (MATRIX MATCHING TYPE QUESTIONS))|1 VideosTHERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions|2 VideosTHERMOMETRY
AAKASH SERIES|Exercise Numerical Exercise (LEVEL- 1)|11 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-THERMODYNAMICS-LECTURE SHEET (EXERCISE - I (INTEGER TYPE QUESTIONS))
- A gas expands in a piston cylinder device from volume V1 to V2, the p...
Text Solution
|
- A 1000kg piston encloses 32g of oxygen gas at a temperature of 27^@C ...
Text Solution
|
- An ideal gas is trapped inside a narrow test tube of area A = 20 xx10...
Text Solution
|
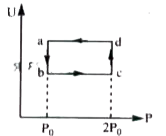
- Figure shows variation of internal energy (U) with the pressure (P) of...
Text Solution
|