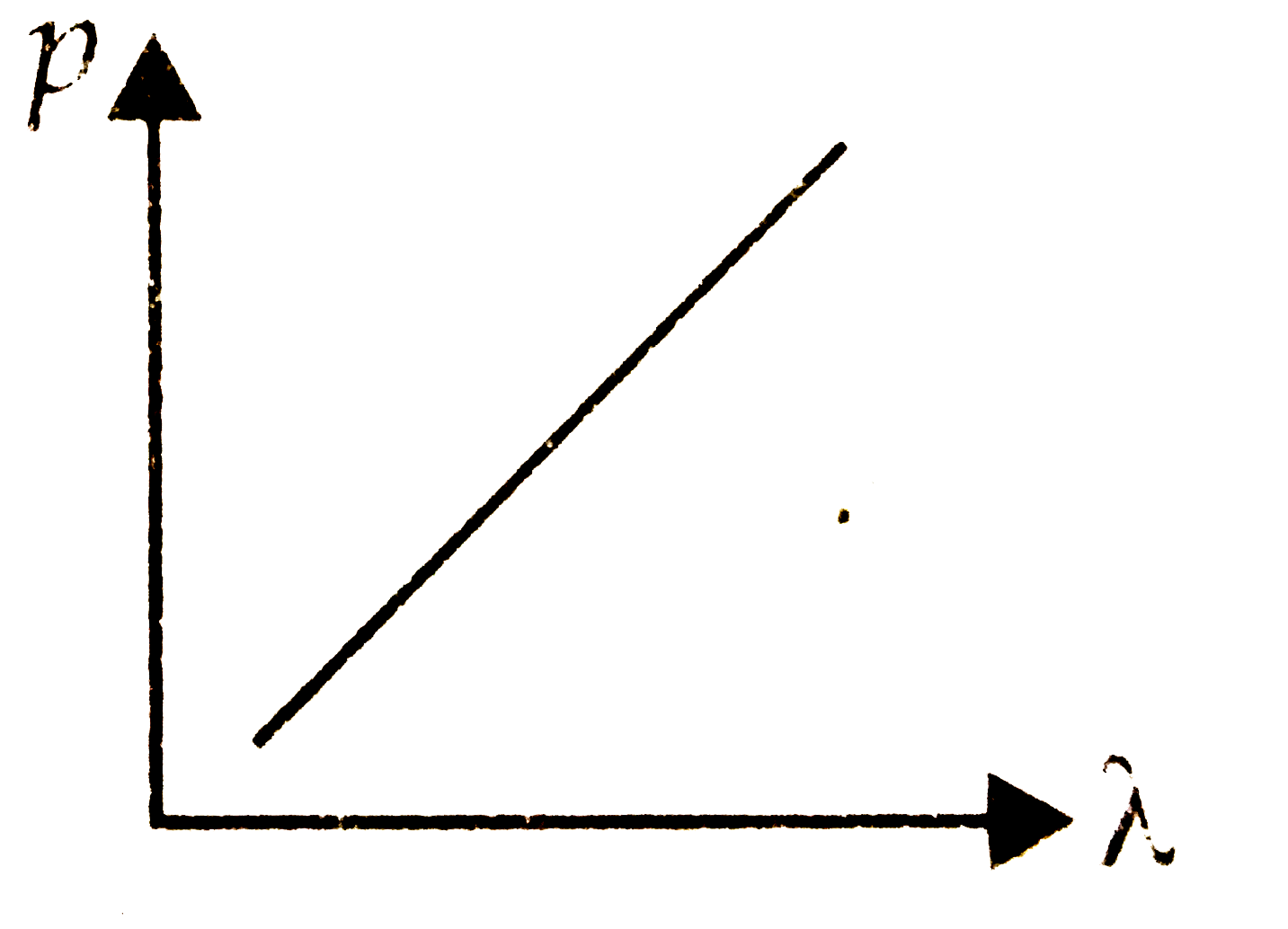
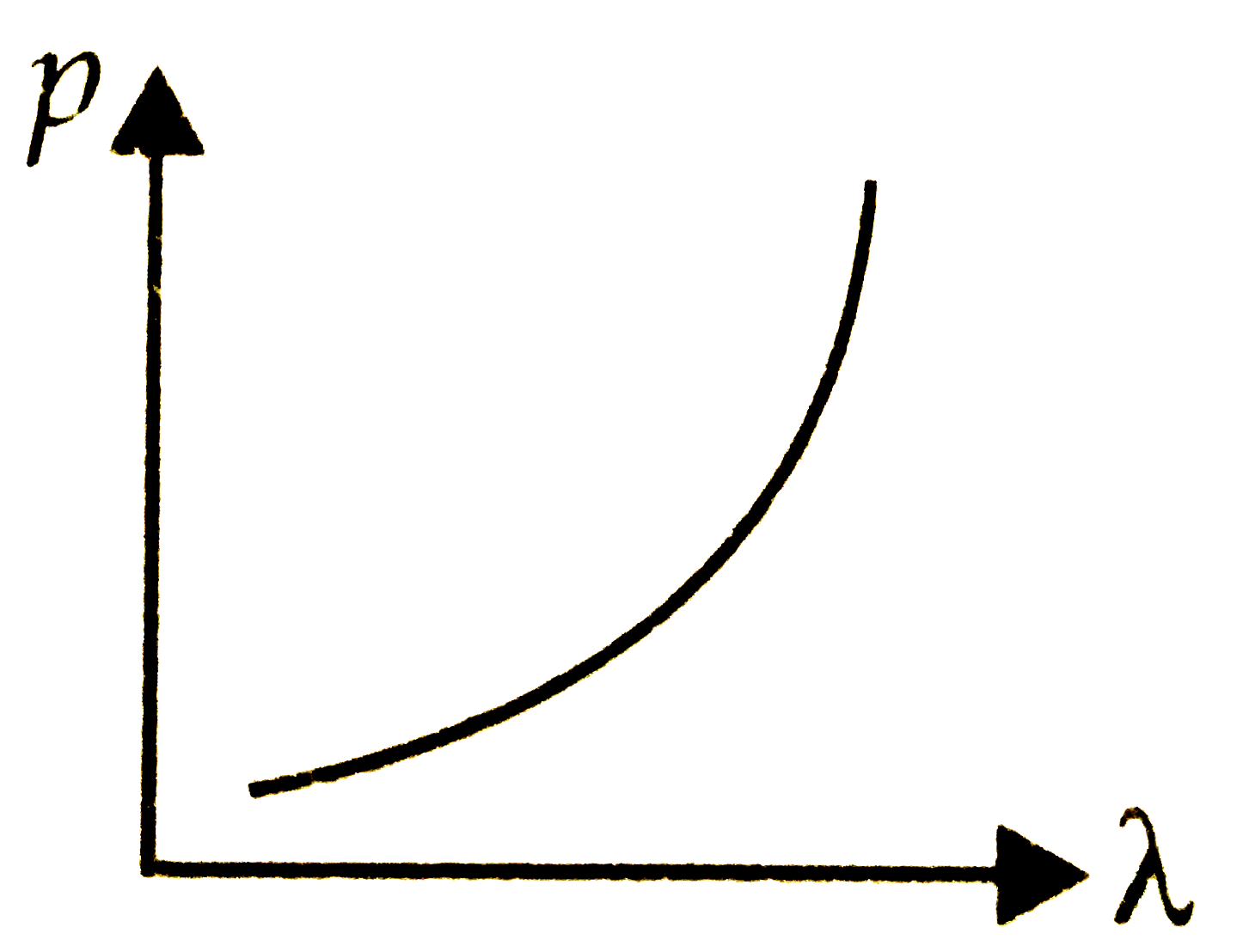
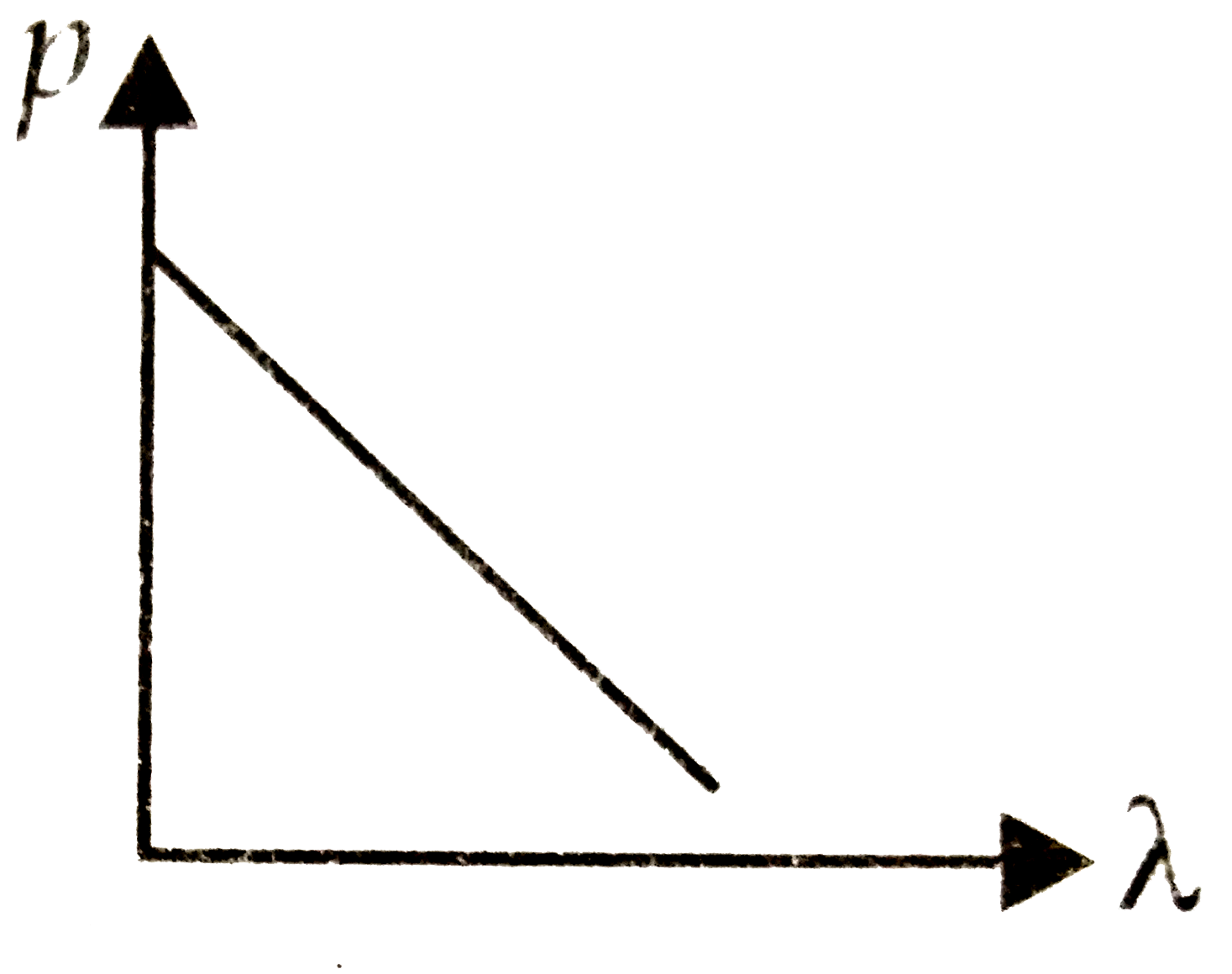
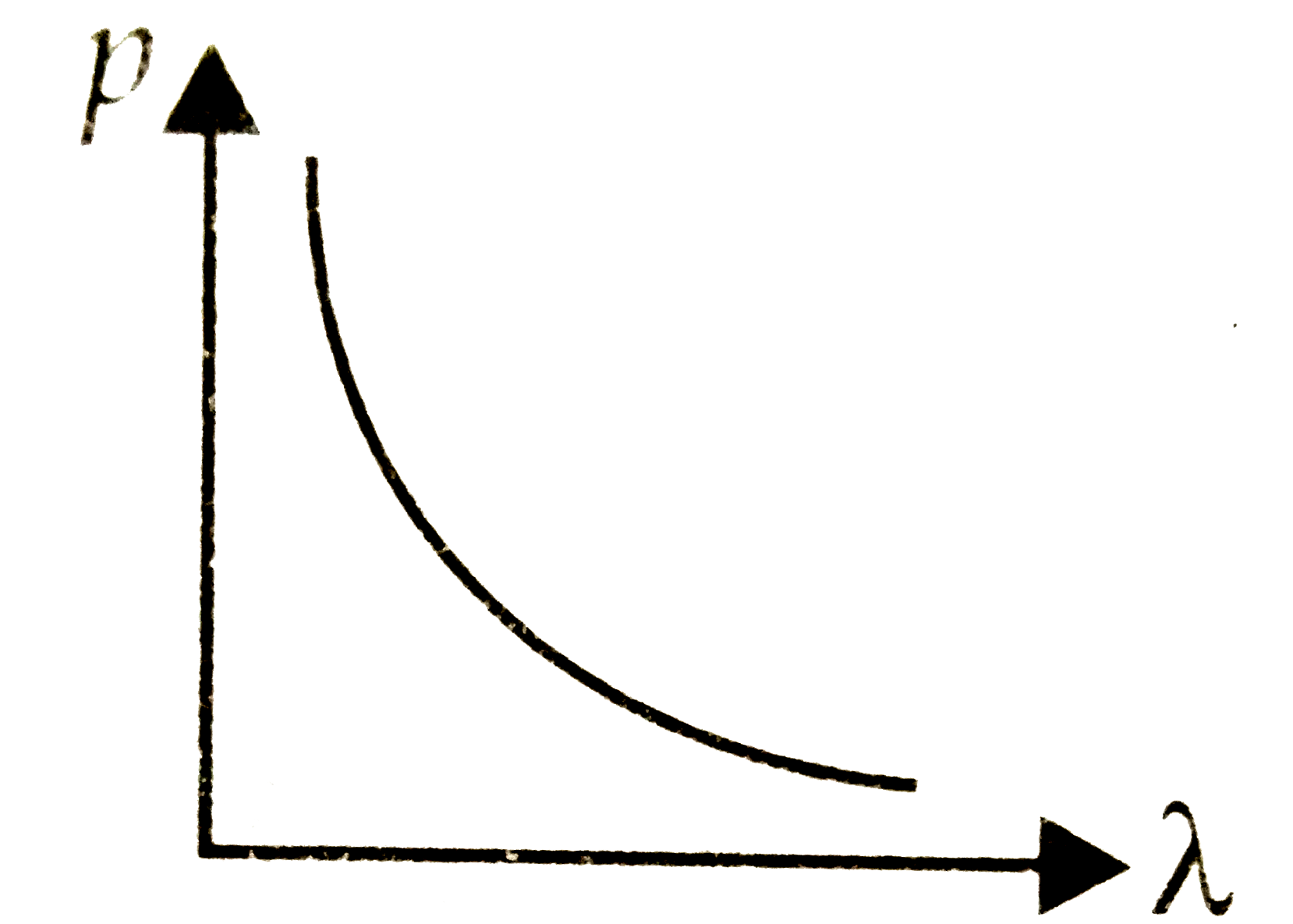
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
NCERT FINGERTIPS ENGLISH|Exercise DAVISSON AND GERMER EXPERIMENT|3 VideosDUAL NATURE OF RADIATION AND MATTER
NCERT FINGERTIPS ENGLISH|Exercise HIGHER ORDER THINKING SKILLS|8 VideosDUAL NATURE OF RADIATION AND MATTER
NCERT FINGERTIPS ENGLISH|Exercise PARTICLE NATURE OF LIGHT : THE PHOTON|19 VideosCURRENT ELECTRICITY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosELECTRIC CHARGES AND FIELDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-DUAL NATURE OF RADIATION AND MATTER -WAVE NATURE OF MATTER
- A particle A with a mass m(A) is moving with a velocity v and hits a p...
Text Solution
|
- The de Broglie wavelength associated with a ball of mass 150 g travell...
Text Solution
|
- Which of the following figure represents the variation of particle mom...
Text Solution
|
- Relativistic corrections become necessary when the expression for the ...
Text Solution
|
- A proton and an alpha - particle are accelerated through same potentia...
Text Solution
|
- The deBroglie wavelength of a particle of kinetic energy K is lamda. W...
Text Solution
|
- When the velocity of an electron increases, its de Broglie wavelength
Text Solution
|
- The de-broglie wavelength of a photon is twice the de-broglie waveleng...
Text Solution
|
- A particle is moving three times as fast as an electron. The ratio of ...
Text Solution
|
- The de Broglie wavelength of an electron with kinetic energy 120 e V i...
Text Solution
|
- The de Broglie wavelength lamda of an electron accelerated through a p...
Text Solution
|
- Assuming an electron is confined to a 1 nm wide region. Find the uncer...
Text Solution
|
- An EM wave of wavelength lambda is incident on a photosensitive surfac...
Text Solution
|
- If the momentum of an electron is changed by p, then the de - Broglie ...
Text Solution
|
- If the kinetic energy of the particle is increased to 16 times its pre...
Text Solution
|
- The de Broglie wavelength of an electron in a metal at 27^(@)C is (...
Text Solution
|
- What is the de-Broglie wavelength of nitrogen molecule in air at 300K ...
Text Solution
|
- A alpha -parhticle moves in a circular path of radius 0.83 cm in th...
Text Solution
|
- Electrons with de-Broglie wavelength lambda fall on the target in an X...
Text Solution
|
- Find the (a) maximum frequency and (b) minimum wave-length of X-rays p...
Text Solution
|