A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEI
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|7 VideosNUCLEI
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosNUCLEI
NCERT FINGERTIPS ENGLISH|Exercise Nuclear Energy|10 VideosMOVING CHARGES AND MAGNETISM
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPRACTICE PAPPER
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|50 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-NUCLEI-Higher Order Thinking Skills
- Sometimes a radioactive nucleus decays into a nucleus which inself is ...
Text Solution
|
- The deuteron is bound by nuclear forces just as H-atom is made up of p...
Text Solution
|
- A fission reaction is given by (92)^(236) U rarr(54)^(140) Xe + (38)^(...
Text Solution
|
- How long can an electric lamp of 100W be kept glowing by fusion of 2.0...
Text Solution
|
- Nuclei of a radioactive element A are being produced at a constant rat...
Text Solution
|
- If alpha=2N0lambda, calculate the number of nuclei of A after one half...
Text Solution
|
- The element curium .96^248 Cm has a mean life of 10^13s. Its primary d...
Text Solution
|
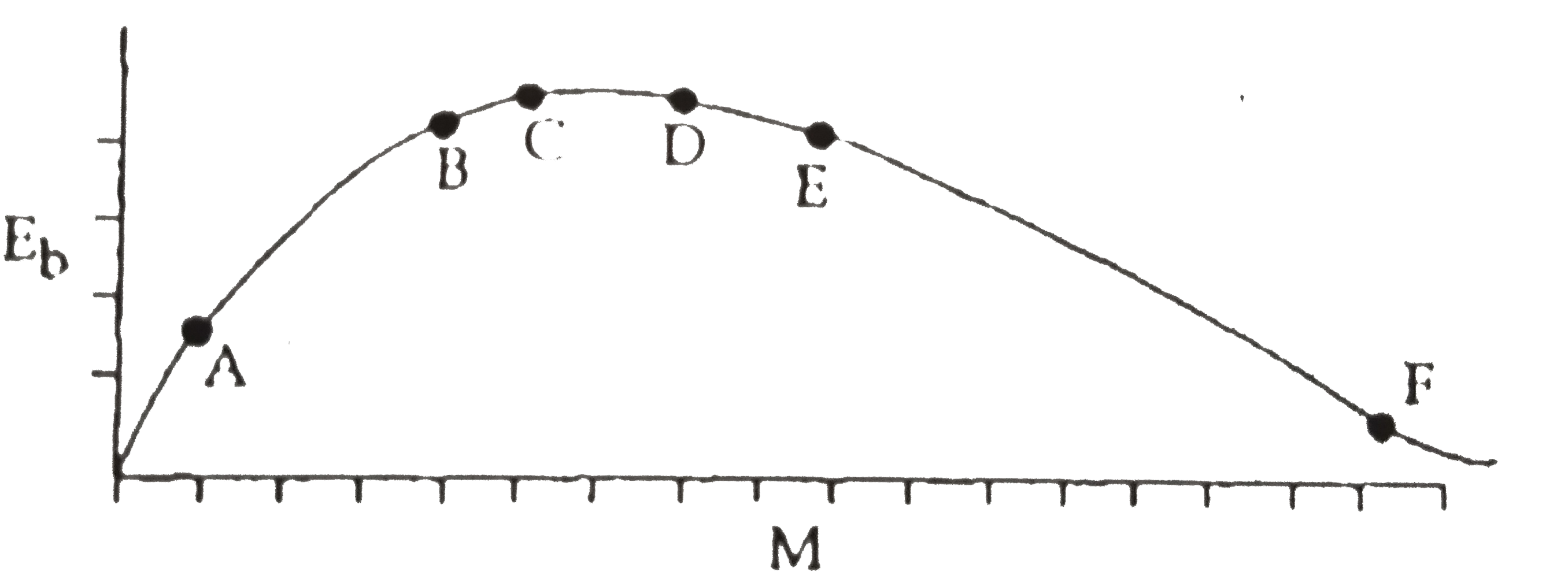
- The above is a plot of binding energy per nucleon E(b), against the nu...
Text Solution
|