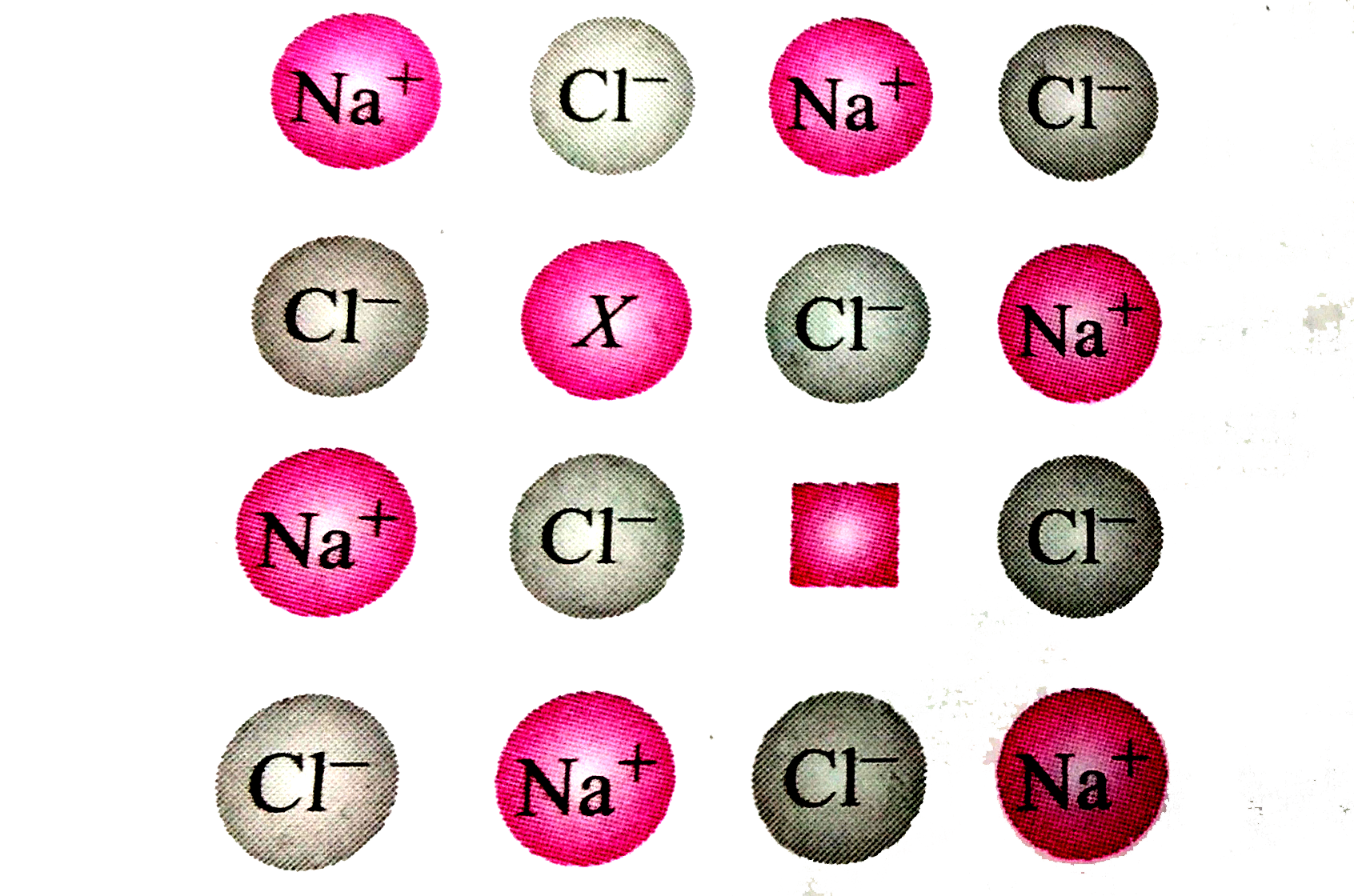A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise Electrical Properties|9 VideosTHE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise Magnetic Properties|5 VideosTHE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise Calculations Involving Unit Cell Dimensions|9 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE SOLID STATE -Imperfections In Solids
- Which is the defect represented by the given figure ?
Text Solution
|
- Due to Frenkel defect, the density of the ionic solids
Text Solution
|
- Why is Frenkel defect not found in pure alkali metal halides ?
Text Solution
|
- Which one of the following crystal does not exhibit Frenkel defect?
Text Solution
|
- What type of stoichiometric defecit is shoen by ZnS ?
Text Solution
|
- In the Schottky defect:
Text Solution
|
- Which of the following defects is represented in the given figure ?
Text Solution
|
- What type of crystal defect is indicated in the diagram given below : ...
Text Solution
|
- The appearance of colour in solid alkali metal halides is generally du...
Text Solution
|
- In the given crystal structure what should be the cation X which repla...
Text Solution
|
- When electron are trapped into the crystal in anion cancy ,the defect ...
Text Solution
|
- In the following figure , the blank X is known as and why ?
Text Solution
|
- The anionic sites occupied by unpaired electrons are called F-centres ...
Text Solution
|
- ZnO shows yellow colour on heating due to
Text Solution
|
- Which of the following set of compounds will show metal deficiency def...
Text Solution
|
- Experimentally it was found that a metal oxide has formula M(0.98)O. M...
Text Solution
|
- Identify A,B,C and D in the following flow chart :
Text Solution
|
- Mark the incorrect pair from the following.
Text Solution
|