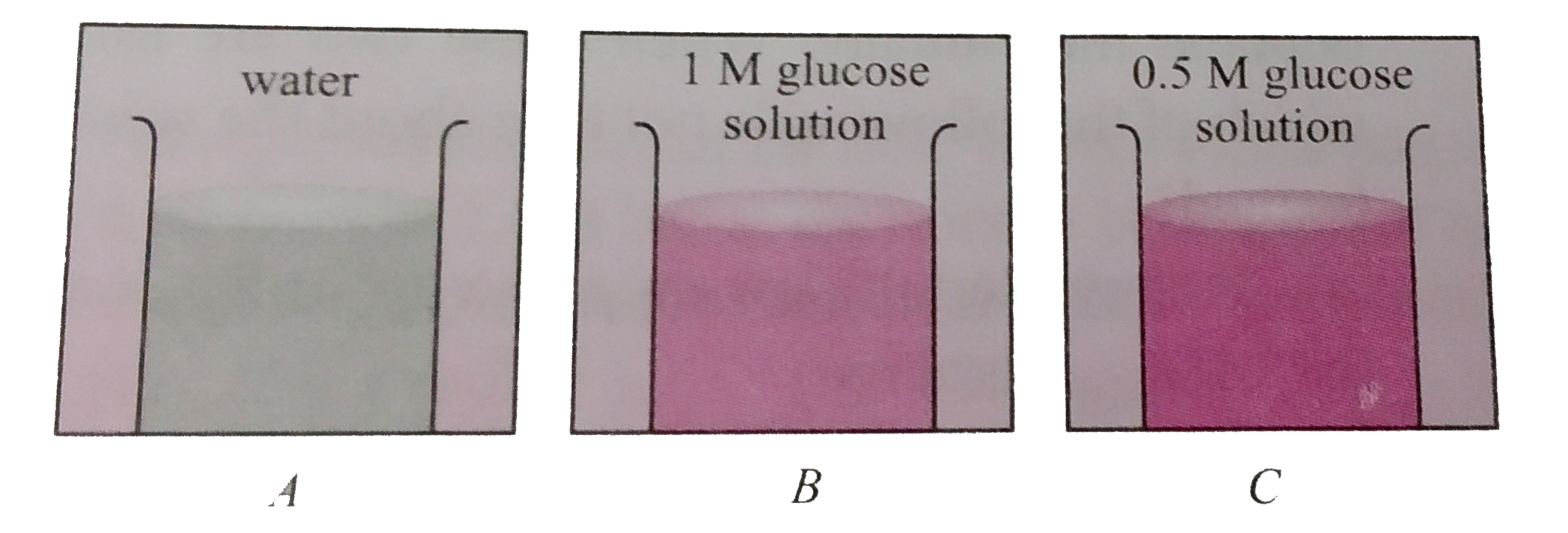
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Ideal And Non-Ideal Solutions|13 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Colligative Properties And Determination Of Molar Mass|32 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Solubility|11 VideosPRACTICE PAPER -3
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|50 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SOLUTIONS -Vapour Pressure Of Liquid Solutions
- Partial pressure of a solution component is directly proportional to i...
Text Solution
|
- 3 moles of P and 2 moles of Q are mixed , what will be their total va...
Text Solution
|
- X,Y and Z in the above graph are
Text Solution
|
- Among the following substances, the lowest vapour pressure is exerted ...
Text Solution
|
- In three beakers labelled as (A),(B) and (C ) , 100 mL of water, 100 m...
Text Solution
|