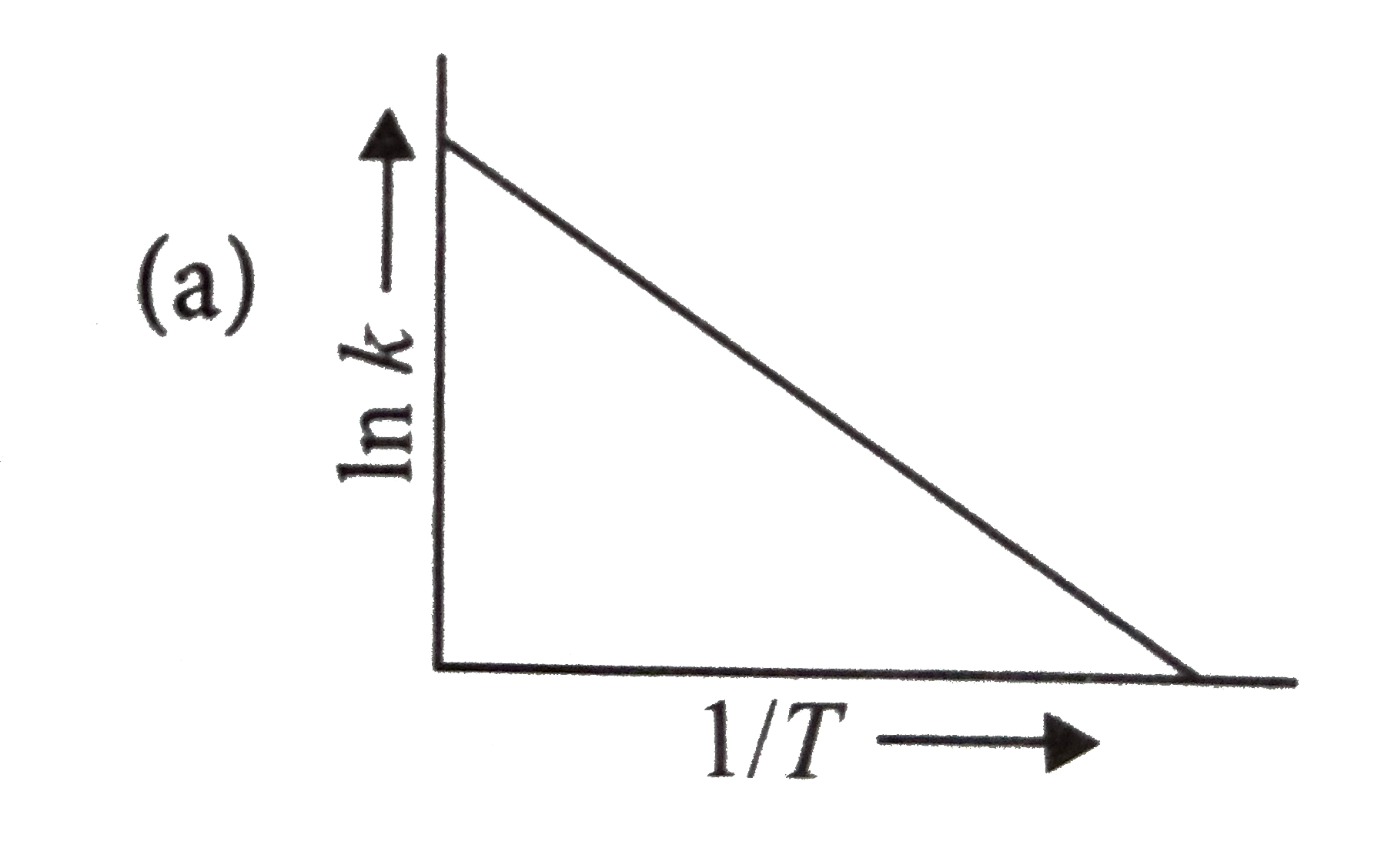
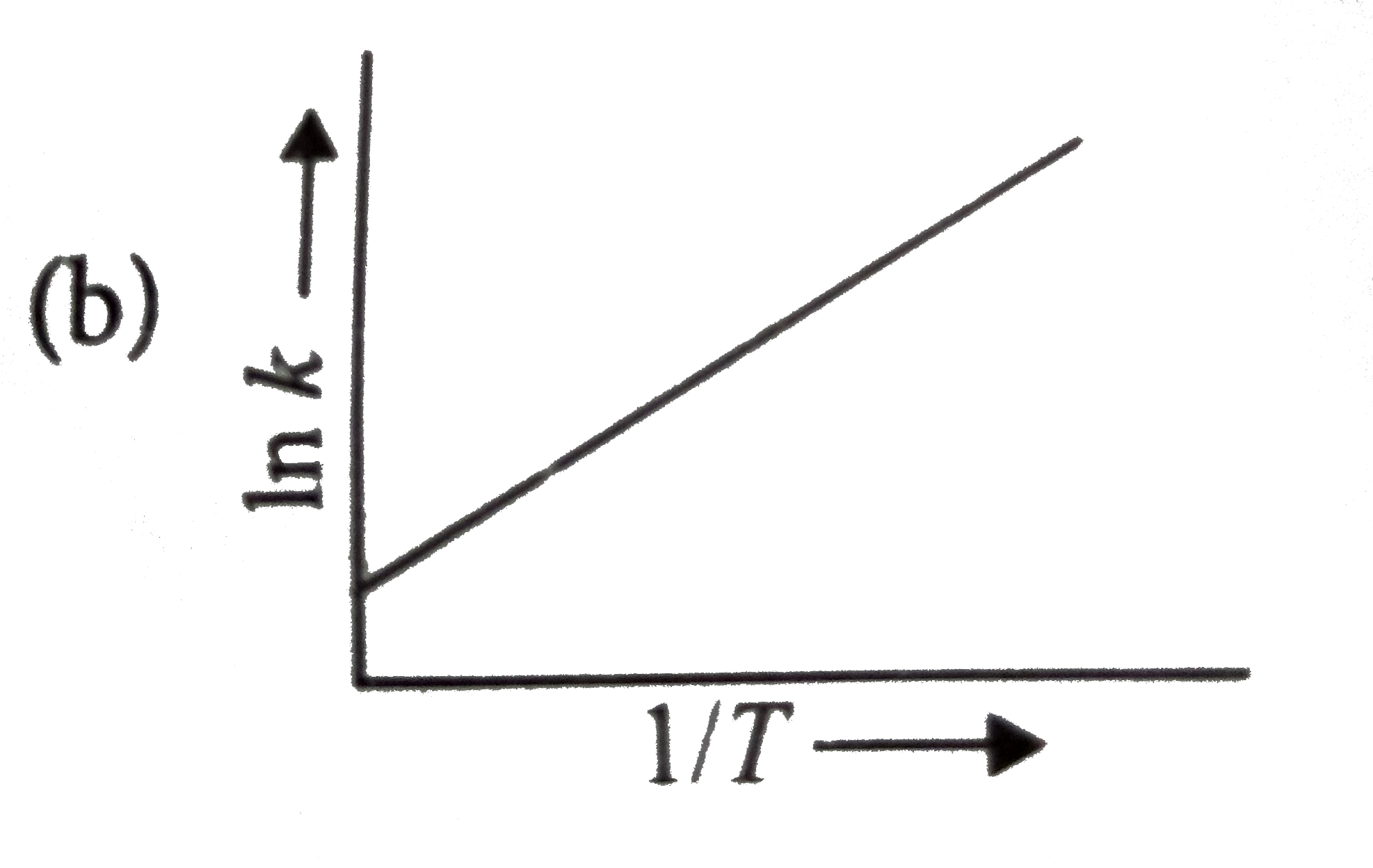
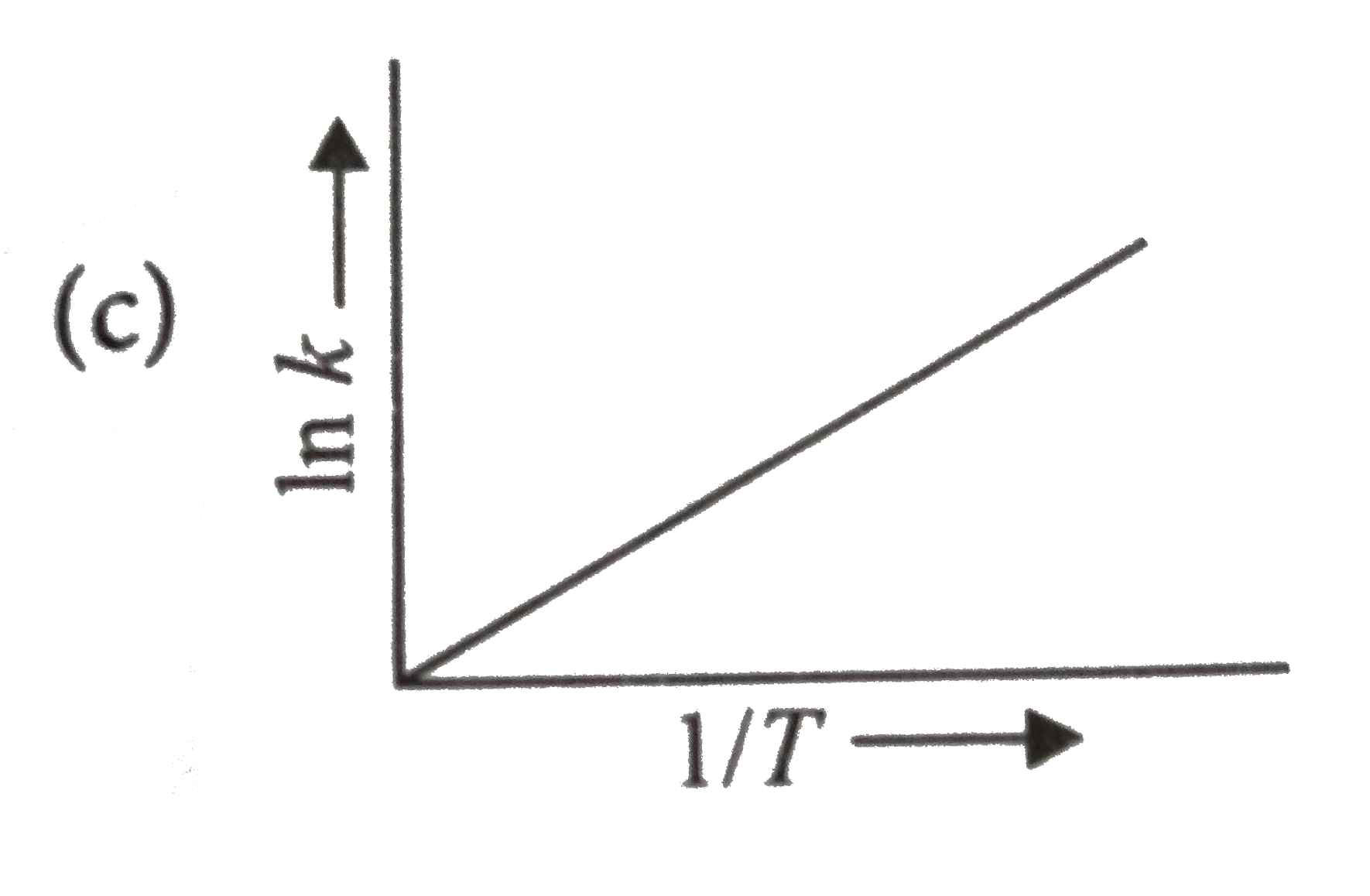
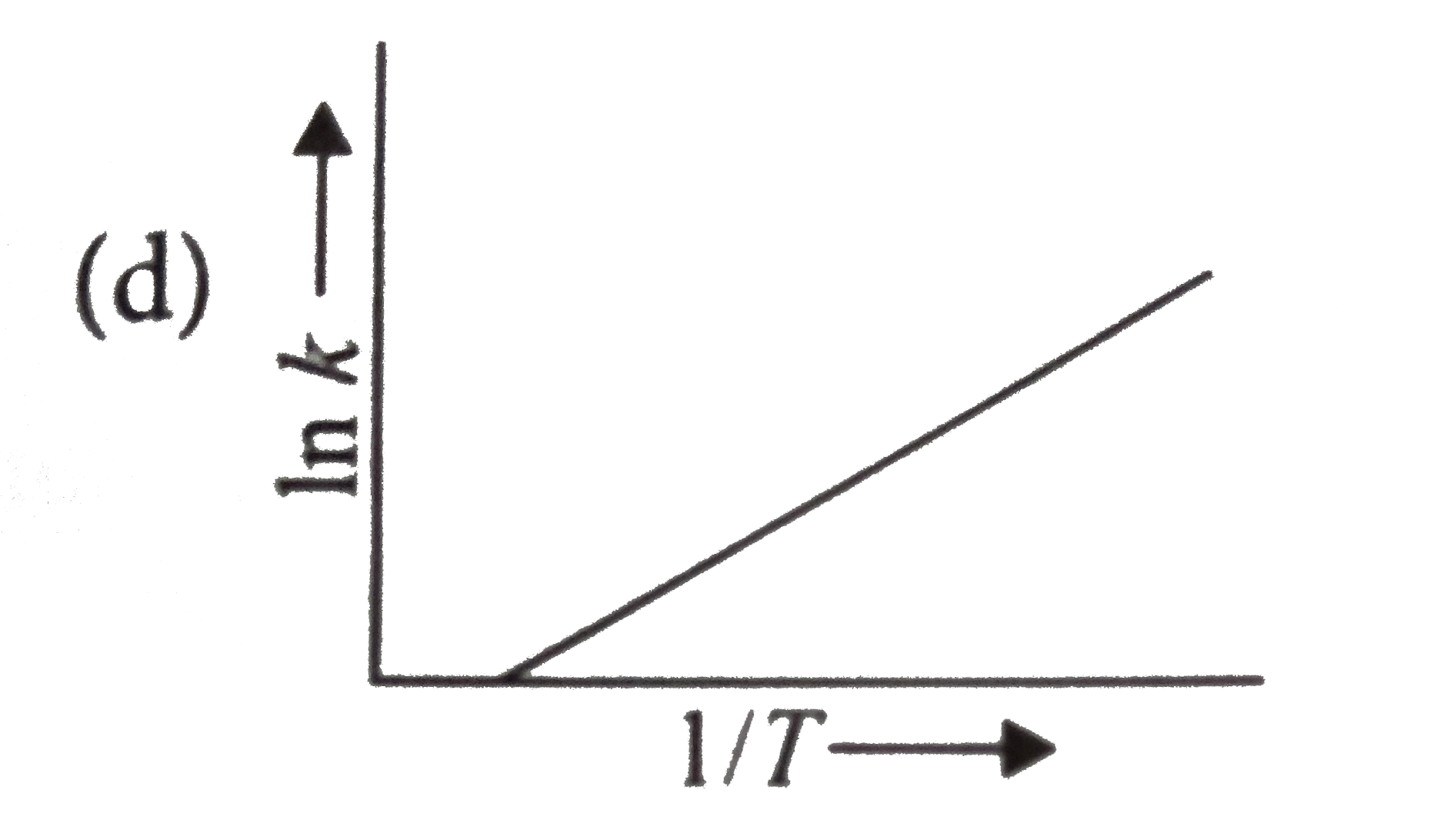
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosCHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise Rate Of A Chemical Reaction|17 VideosCHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinking Skills|9 VideosBIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-CHEMICAL KINETICS-NCERT EXEMPLAR PROBLEMS
- The role of a catalyst is to change
Text Solution
|
- In the presence of a catalyst, the heat evolved or absorbed during the...
Text Solution
|
- Activation energy of a chemical reaction can be determined by
Text Solution
|
- Consider a first order gas phase decomposition reaction given below: ...
Text Solution
|
- According to Arrhenius equation rate constant k is equal to Ae^(-E(a)/...
Text Solution
|
- Consider the Arrhenius equation given below and mark the correct op...
Text Solution
|
- Which of the following statement is not correct about order of a ...
Text Solution
|
- Consider the graph given in Q.9 Which of the following options does n...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following expression is correct for the rate of reac...
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- Which of the following statements is incorrect about the collison theo...
Text Solution
|
- A first order reaction is 50% completed in 1.26 xx 10^(14)s. How much ...
Text Solution
|
- Compound 'A' and B react according to the following chemical equation....
Text Solution
|
- Which of the following statements is not correct for the catalyst?
Text Solution
|
- The value of rate constant of a pseudo first order reaction
Text Solution
|
- Consider the reaction A to B. The concentration of both the reactan...
Text Solution
|