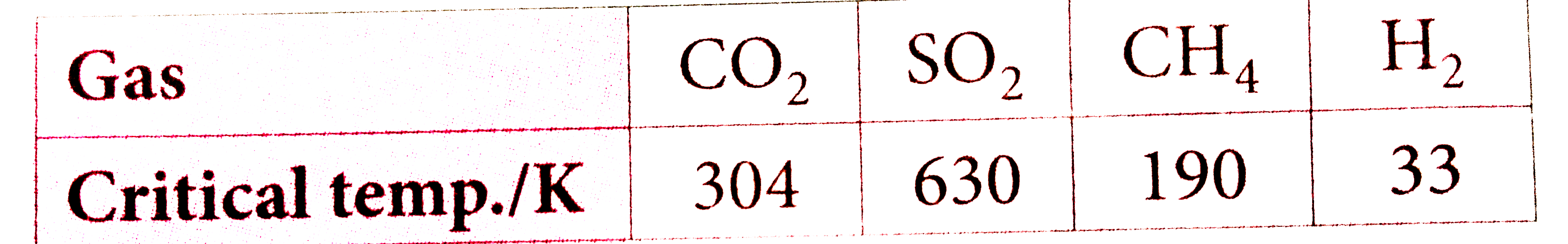A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Adsorption|26 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SURFACE CHEMISTRY-NCERT EXEMPLAR PROBLEMS
- Extent of adsorption of adsorbate from solution phase increases with ...
Text Solution
|
- Which one of the following is not applicable to the phenomenon of adso...
Text Solution
|
- Which of the following is not favourable condition for physical adsorp...
Text Solution
|
- Physical adsorption of a gaseous species may change to chemical adsorp...
Text Solution
|
- In physisorption adsorbent does not show specificity for any particula...
Text Solution
|
- Which of the following is an example of absorption?
Text Solution
|
- On the basis of data below predict which of the following gases shows ...
Text Solution
|
- In which of the following reactions heterogeneous catalysis is involve...
Text Solution
|
- At high concentration of soap in water, soap behaves as …… .
Text Solution
|
- Which of the following will show Tyndall effect?
Text Solution
|
- Method by which lyophobic sol can be protected
Text Solution
|
- Freshly prepared precipitate sometimes gets converted to colloidal sol...
Text Solution
|
- Which of the following electrolytes will have maximum coagulating valu...
Text Solution
|
- A colloidal system having a solid substance as a dispersed phase and a...
Text Solution
|
- The values of colligative properties of colloidal solution are of smal...
Text Solution
|
- Arrange the following diagrams in correct sequence of steps involved i...
Text Solution
|
- Which of the following process is responsible for the formation of del...
Text Solution
|
- Which of the following curves is in accordance with Freundlich adsorpt...
Text Solution
|
- Which of the following process is not responsible for the presence of ...
Text Solution
|
- Which of the following phenomenon is applicable to the process shown i...
Text Solution
|