A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise EXAMPLAR PROBLEMS|25 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE P-BLOCK ELEMENTS -Assertion And Reason
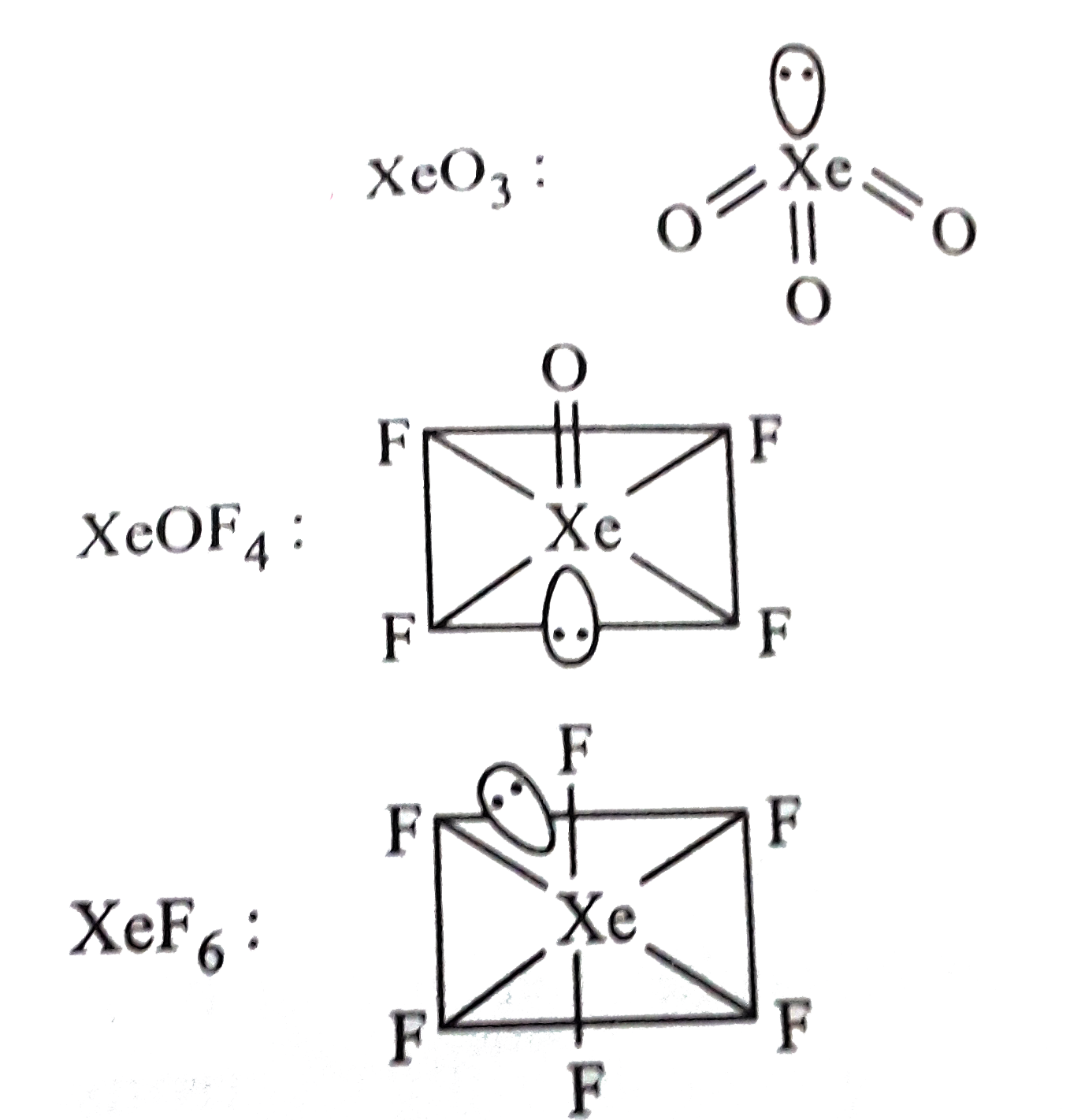
- Among the following molecules (i) XeO(3) (ii) XeOF(4) (iii) XeF(6) ...
Text Solution
|
- Assertion : The covalence of nitrogen in N(2)O(5) is 5 Reason : Nitr...
Text Solution
|
- Assertion : Catenation tendency is weaker in nitrogen. Reason : Nitr...
Text Solution
|
- Assertion: Ammonia acts as a ligand. Reason : A lone pair of electro...
Text Solution
|
- Assertion : White phosphorus is more reactive than red phosphorus. R...
Text Solution
|
- Assertion : In trigonal bipyramidal structure two axial bonds are long...
Text Solution
|
- Assertion : Acidic character of group 16 hydrides increases from H(2)O...
Text Solution
|
- Assertion : Ozone layer in the upper region of atmosphere protects ear...
Text Solution
|
- Assertion : O(3) acts as a powerful oxidising agent. Reason : O(3) o...
Text Solution
|
- Assertion: In vapour state sulphur is paramagnetic in nature. Reason...
Text Solution
|
- Assertion : Sulphuric acid reacts with sodium chloride in the followin...
Text Solution
|
- Assertion : Fluorine combines with sulphur to form SF(6) but no other ...
Text Solution
|
- Assertion : Fluorine oxidises water to oxygen whereas chlorine and bro...
Text Solution
|
- Assertion: HClO(4) is a stronger acid than HClO(3). Reason: Oxidatio...
Text Solution
|
- Assertion : Interhalogen compounds are more reactive than halogens (ex...
Text Solution
|
- Assertion :Solubility of noble gases in water decreases with increases...
Text Solution
|