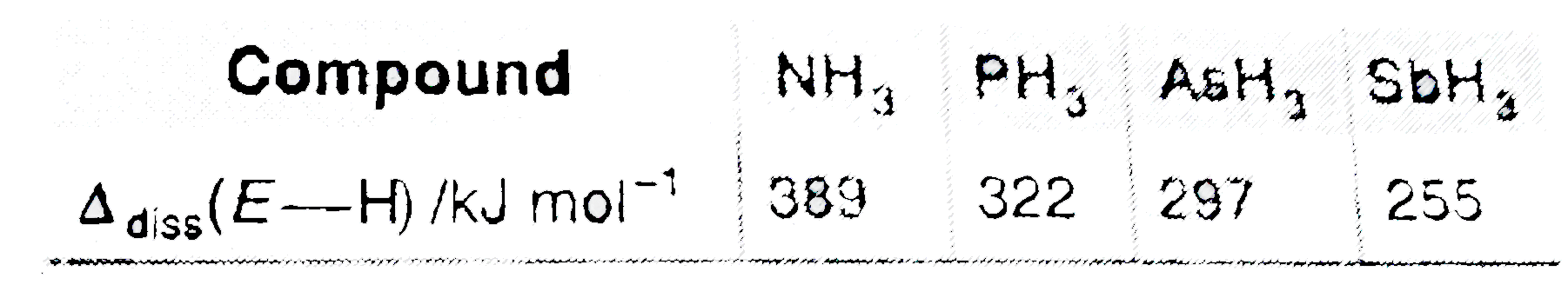A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinking Skills|7 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE P-BLOCK ELEMENTS -NCERT Exemplar
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Affinity for hydrogen decreases in the group from fluorine to iodine. ...
Text Solution
|
- Bond dissociation enthalpy of E-H (E=element) bond is given below. ...
Text Solution
|
- On heating with concentrated NaOH solution in an inert atmosphere of C...
Text Solution
|
- Which of the following acids forms three series of salts?
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- On heating lead nitrate forms oxides of nitrogen and lead. The oxides ...
Text Solution
|
- Which of the following elements does not show allotropy ?
Text Solution
|
- Maximum covalency of nitrogen is :
Text Solution
|
- Which of the following statements is wrong?
Text Solution
|
- A brown ring is formed in the ring test for NO(3)^(-) ion. It is due t...
Text Solution
|
- Elements of group 15 form compounds in +5 oxidatin state. However, bis...
Text Solution
|
- On heating ammonium dichromate and barium azide separately we get
Text Solution
|
- In the preparation of HNO(3), we get NO gas by catalytic oxidation of ...
Text Solution
|
- The oxidation state of central atom in the anion of compound NaH(2)PO(...
Text Solution
|
- Which of the following is not tetrahedral in shape ?
Text Solution
|
- Which of the following are peroxoacids of sulphur ?
Text Solution
|
- Hot conc. H(2)SO(4) acts as moderately strong oxidising agent. It oxid...
Text Solution
|
- A black compound of manganese reacts with a halogen acid to give green...
Text Solution
|
- In the preparation of compounds of Xe, Bartlett had taken O(2)^(+) Pt ...
Text Solution
|