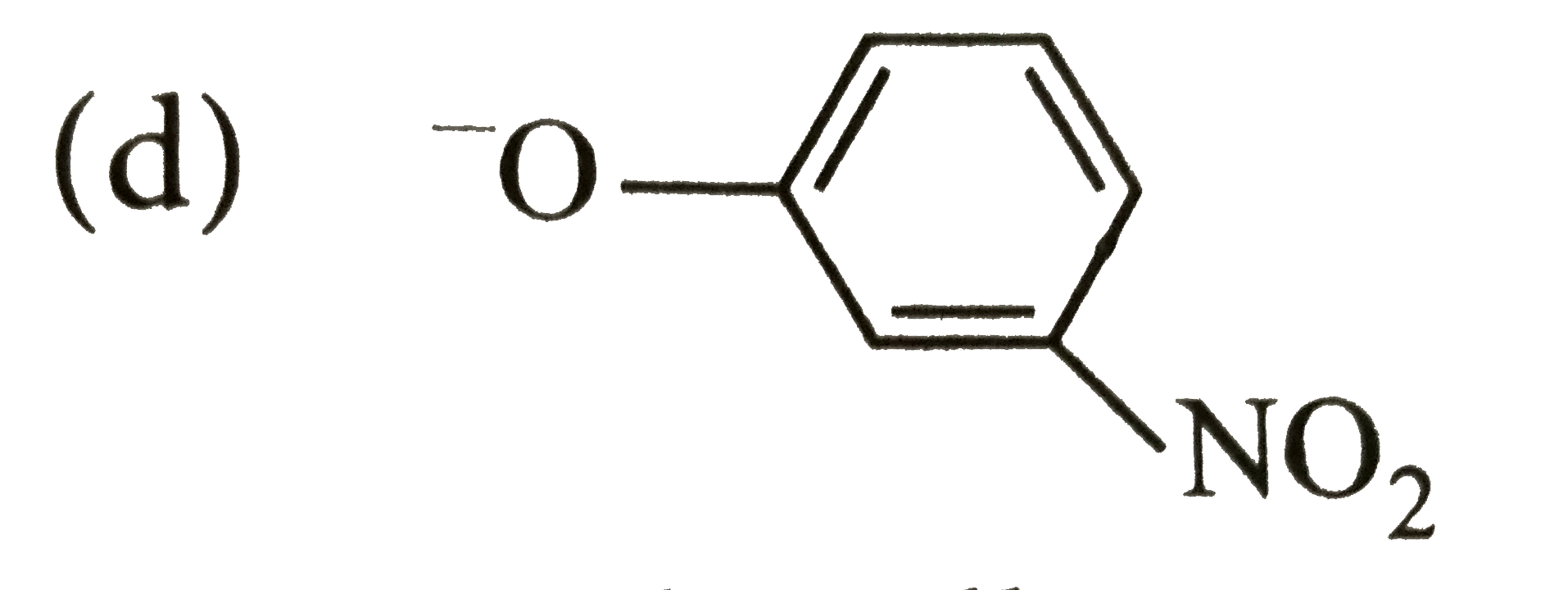
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise Classification|2 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise Nomenclature|3 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ALCOHOLS,PHENOLS AND ETHERS-EXEMPLAR PROBLEMS
- Monochlorination of toluene in sunlight followed by hydroysis with aq....
Text Solution
|
- How many alcohols with molecular formula C(4)H(10)O are chiral in natu...
Text Solution
|
- What is the correct order of rectivity of alchohols in the following r...
Text Solution
|
- CH3CH2OH can be converted into CH3CHO by
Text Solution
|
- The process of converting alkyl halides into alcohols involves...........
Text Solution
|
- Give IUPAC name of the compund given below: CH3-underset(Cl)underse...
Text Solution
|
- IUPAC name of m-cresol is.......... .
Text Solution
|
- IUPAC name of the compund CH3-underset(CH3)underset(|)"CH"-OCH3is
Text Solution
|
- Which of the following species can act as the strongest base ?
Text Solution
|
- Which of the following compounds will react with sodium hydroxide solu...
Text Solution
|
- Phenol is less acidic than
Text Solution
|
- Which of the following is most acidic?
Text Solution
|
- Mark the correct order of decresing acid strenght of the following com...
Text Solution
|
- Arrange the following compounds in increasing order of boiling point :...
Text Solution
|