A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Mole Concept And Molecular Masses|23 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Percentage Composition|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Laws Of Chemical Combination|13 VideosREDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosSTATES OF MATTER
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SOME BASIC CONCEPTS OF CHEMISTRY -Atomic And Molecular Masses
- The reference standard used for defining atomic mass is
Text Solution
|
- Are the atomic masses of some elements actually fractional ?
Text Solution
|
- Oxygen occurs in nature as a mixture of isotopes ""^16O, ""^17O " and ...
Text Solution
|
- For every one ""^37Cl isotopes there are three ""^35Cl isotopes in a s...
Text Solution
|
- Carbon occur in nature as a mixture of C-12 and C-13. Average atomic m...
Text Solution
|
- Packing of Na^+ " and " Cl^- ions in sodium chloride is depicted by th...
Text Solution
|
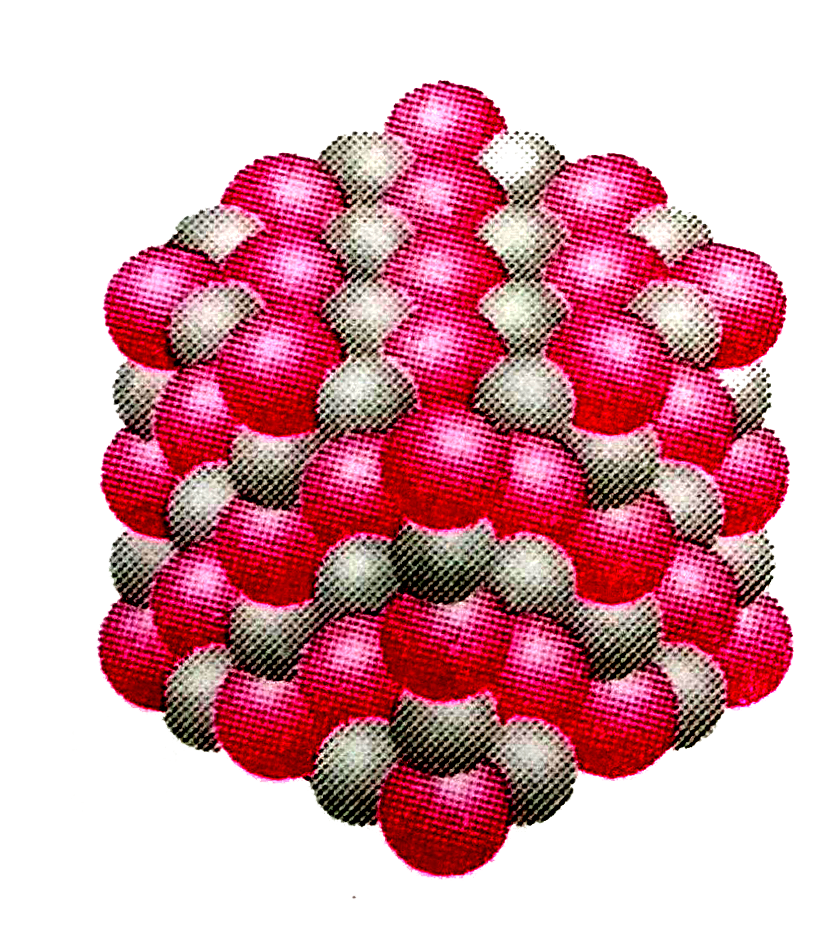 (A) in solid state, sodium chloride does not exist as a single entity
(B) formula mass of NaCl is 68.0u
(C) formula mass of NaCl is the sum of atomic masses of Na and Cl
(D) both A and C
(A) in solid state, sodium chloride does not exist as a single entity
(B) formula mass of NaCl is 68.0u
(C) formula mass of NaCl is the sum of atomic masses of Na and Cl
(D) both A and C