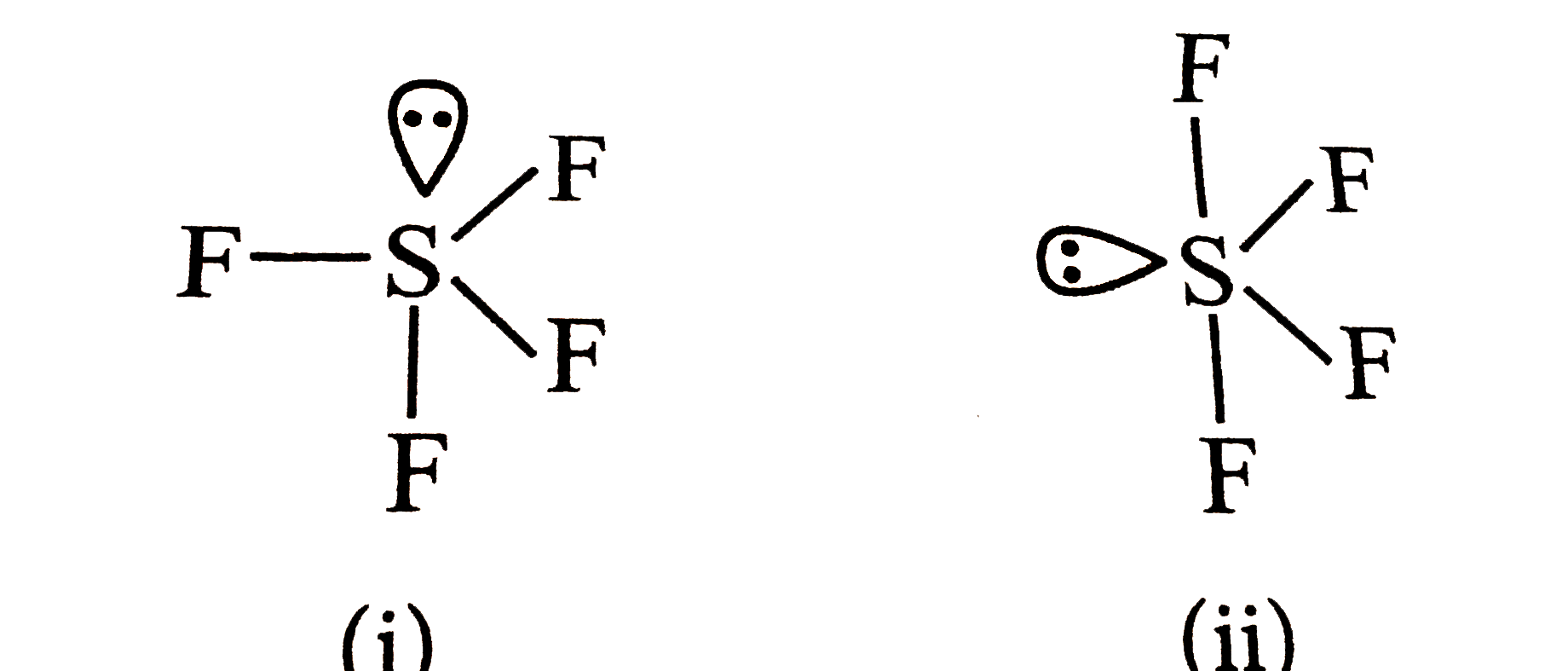A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS ENGLISH|Exercise Valence Bond Theory|7 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS ENGLISH|Exercise Hybridisation|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS ENGLISH|Exercise Bond Parameters|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-CHEMICAL BONDING & MOLECULAR STRUCTURE-Vsepr Theory
- According to VSEPR THEORY
Text Solution
|
- In a bonded molecule, the order of repulsion between the bonded and no...
Text Solution
|
- In water molecule, the bond angle of 104.5^(@) around oxygen is accoun...
Text Solution
|
- Which of the following shapes of SF(4) is more stable and why?
Text Solution
|
- The most stable shape of ClF(3) is shown by
Text Solution
|
- Which of the following acts as a ligand but does not have any lone pai...
Text Solution
|
- Few examples of the componds formed by chemical bonding are given belo...
Text Solution
|
- Which of the following statements is correct regarding the structure o...
Text Solution
|
- Match the molecules given in column I with their shapes given in colum...
Text Solution
|
- Which of the following are iso-electronic as well as iso-structural ? ...
Text Solution
|
- Which molecule is depicted by the given ball and stick models ?
Text Solution
|
- Given below is the table showing shapes of some molecules having lone ...
Text Solution
|
- Which of the following does not show octahedral geometry ?
Text Solution
|
- The BCl3 is a planar molecule whereas NCl3 is pyramidal because
Text Solution
|
- CF(4), SF(4) and XeF(4) contain the following electronic structure on ...
Text Solution
|