A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Spontaneity|30 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Gibbs Energy Change And Equilibrium|4 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Enthalpy Change Of A Reaction|13 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THERMODYNAMICS-Enthalpies For Different Type Of Reaction
- What will be the amount of heat evolved by burning 10 L of methane und...
Text Solution
|
- For a reaction, C((s))+O(2(g))rarrCO(2(g)) What is the relation betw...
Text Solution
|
- The heat of combustion of C, S and CS(2) are -393.3kJ, -293.7kJ and -1...
Text Solution
|
- How much heat is evolved if 3.2 g of methane is burnt and if the heat ...
Text Solution
|
- What will be the enthalpy of formation of carbon to produce carbon mon...
Text Solution
|
- Two reactions are given below : C("(graphite)")+O(2(g))rarrCO(2(g)),...
Text Solution
|
- Which of the following relationships is not correct ?
Text Solution
|
- Which of the following is not a correct statement about enthalpy of so...
Text Solution
|
- The enthalpy of solution of sodium chloride is 4 kJ mol^(-1) and its e...
Text Solution
|
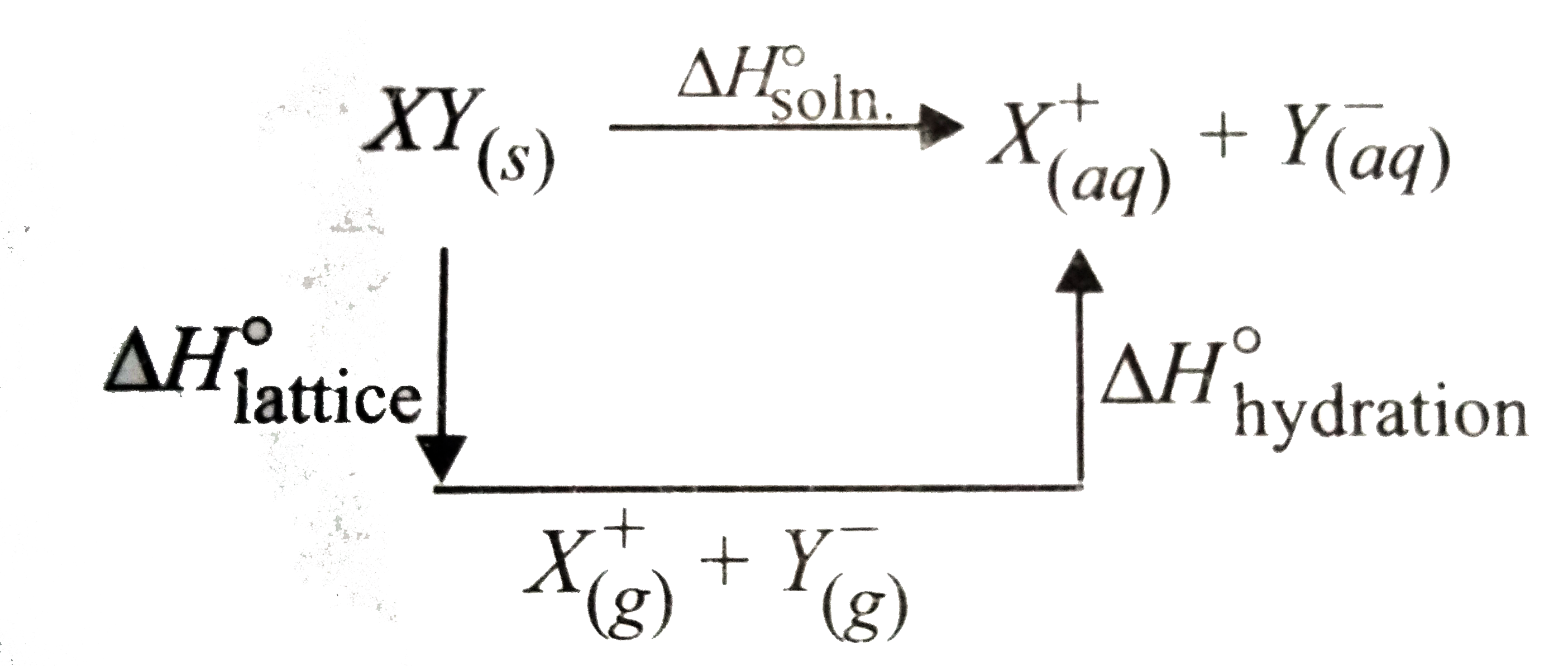
- Study the figure given below and mark the correct expression. The...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- The amount of heat evolved when 0.50 mole of HCl is mixed with 0.30 mo...
Text Solution
|
- Which of the following reactions will have the value of enthalpy of ne...
Text Solution
|
- Bond energies of H-H and Cl-Cl are "430 kJ mol"^(-1) and "242 kJ mol"^...
Text Solution
|
- What will be DeltaH for the reaction, CH(2)Cl(2)rarrC+2H+2Cl? (B.E...
Text Solution
|
- DeltaH for the reaction, OF(2)+H(2)OrarrO(2)+2HF (B.E. of O-F, O-H, ...
Text Solution
|
- Dissociation of water takes place in two steps : H(2)OrarrH+OH," ...
Text Solution
|
- Bond energies of few bonds are given below : Cl-Cl="242.8 kJ mol"^(-...
Text Solution
|
- Which is the correct order of bond energy of single, double and triple...
Text Solution
|
- Which thermochemical process is shown by the following figure?
Text Solution
|