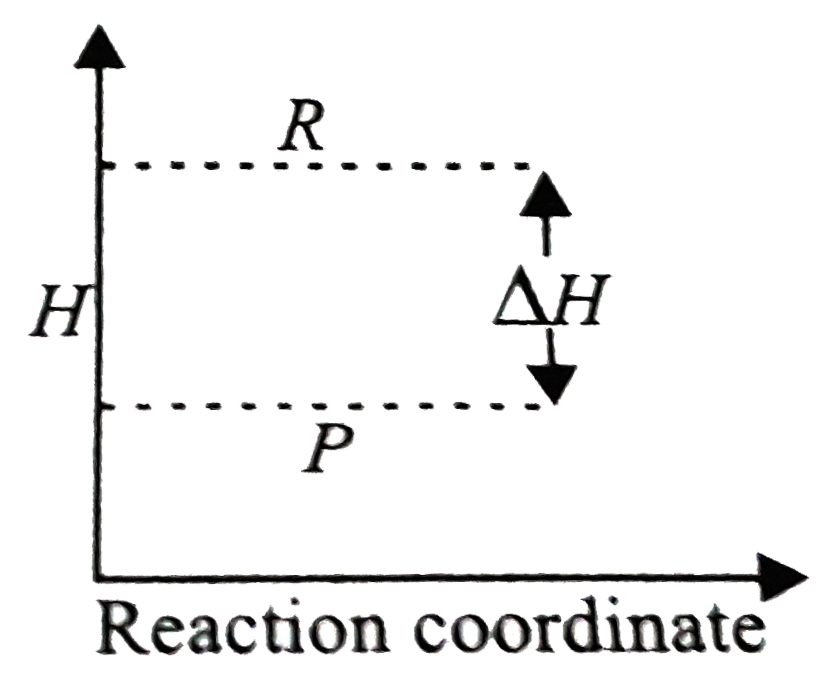A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Gibbs Energy Change And Equilibrium|4 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|11 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Enthalpies For Different Type Of Reaction|22 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THERMODYNAMICS-Spontaneity
- The given enthalpy diagram reperesents which of the following reaction...
Text Solution
|
- Study the given graph and choose the correct option.
Text Solution
|
- In endothermic reactions,
Text Solution
|
- The total entropy change (DeltaS("total")) for the system and surround...
Text Solution
|
- Which of the following expressions regarding entropy is not correct?
Text Solution
|
- For a reaction, P+Q rarr R+S. The value of DeltaH^(@) is -"30 kJ mol"^...
Text Solution
|
- Which of the following reactions will have the value of DeltaS with a ...
Text Solution
|
- A reaction is at equilibrium at 100^(@)C and the enthalpy change for t...
Text Solution
|
- Enthalpy change for the process, H(2)O"(ice")hArr H(2)O"(water)" i...
Text Solution
|
- At what temperature liquid water will be in equilibrium with water vap...
Text Solution
|
- For a reaction: X rarr Y+Z Absolute entropies are X="120 J K"^(-1)"m...
Text Solution
|
- What will be the melting point of KCl if enthalpy change for the reac...
Text Solution
|
- For reversible reaction : X((g))+3Y((g))hArr 2Z((g)), DeltaH=-"40 kJ" ...
Text Solution
|
- At 373 K, steam and water are in equilibrium and DeltaH=40.98" kJ mol"...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- Read the following statements regarding spontaneity of a process and m...
Text Solution
|
- Which of the following processes is a non-spontaneous process?
Text Solution
|
- Which of the following statements regarding Gibb's energy change is co...
Text Solution
|
- For a reaction to be spontaneous at any temperature, the conditions ar...
Text Solution
|
- For the reaction given below the value of standard Gibbs free energy o...
Text Solution
|