A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|11 VideosREDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosREDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise Oxidation Number|70 VideosPRACTICE PAPER 3
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|46 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|11 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-REDOX REACTIONS-Redox Reactions And Electrode Processes
- What will be the products of electrolysis of AgNO(3) solution in water...
Text Solution
|
- In an oxidation proces for a cell, M(1)rarr M(1)^(n+)+n e^(-), t...
Text Solution
|
- Which of the following reactions takes place at anode ?
Text Solution
|
- Which of the following will act as cathode when connected to standard ...
Text Solution
|
- Which of the following reaction does not take place at cathode ?
Text Solution
|
- Based on the following reactions, arrange the metals in increasing ord...
Text Solution
|
- Which of the following is not a correct statement about electrochemica...
Text Solution
|
- If a spoon of copper metal is placed in a solution of FeSO(4), what wi...
Text Solution
|
- The solution in a beaker turns blue if
Text Solution
|
- The standard electrode potential a Ag^(+)//Ag is +0.80 V and of Cu^(2+...
Text Solution
|
- The E^(@) values of redox complex of halogens are given. Based on thes...
Text Solution
|
- Arrange the following metals in the order in which they displace easy ...
Text Solution
|
- Arrange the following metals in increasing order of their reducing po...
Text Solution
|
- A metal X displaces nickel from nickel sulphate solution but does not ...
Text Solution
|
- Given E(Ag^(+)//Ag)^(@)=+0.80 V, E(Cu^(2+)//Cu)^(@)=+0.34 V, E(Fe^(3+)...
Text Solution
|
- निम्नलिखित आयनो को इलेक्ट्रॉन ग्रहण करने की बढ़ती क्षमता के क्रम में लि...
Text Solution
|
- What will be the order of decreasing reducing nature for the given met...
Text Solution
|
- Which of the following is the strongest oxidizing agent ?
Text Solution
|
- Fluorine is the best oxidising agent because it has
Text Solution
|
- Which of the following halides is most acidic ?
Text Solution
|
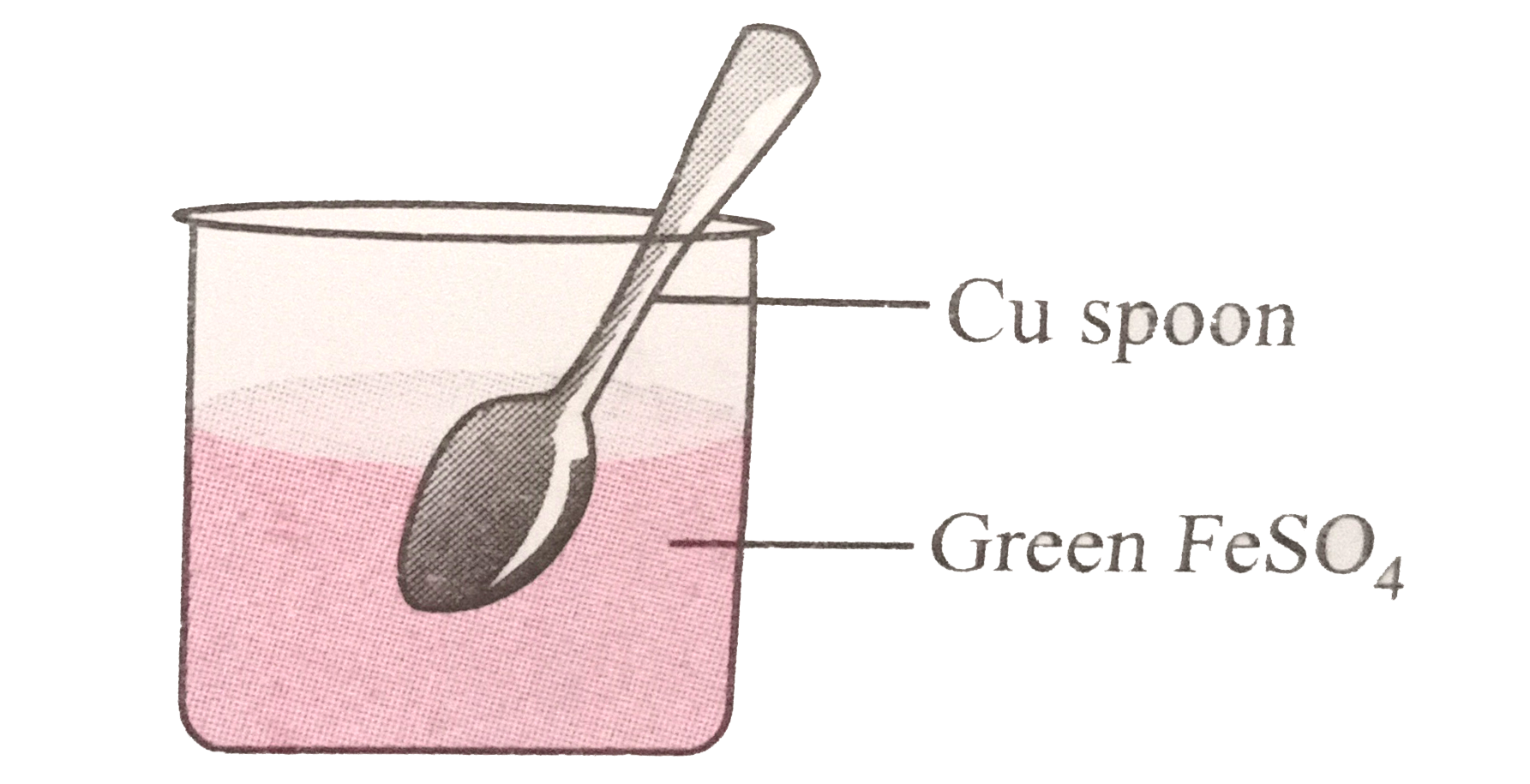 (A)Copper is dissolved in
F
e
S
O
4
to give brown deposit.
(B)No reaction takes place.
(C)Iron is deposited on copper spoon.
(D)Both copper and iron are precipitated
(A)Copper is dissolved in
F
e
S
O
4
to give brown deposit.
(B)No reaction takes place.
(C)Iron is deposited on copper spoon.
(D)Both copper and iron are precipitated