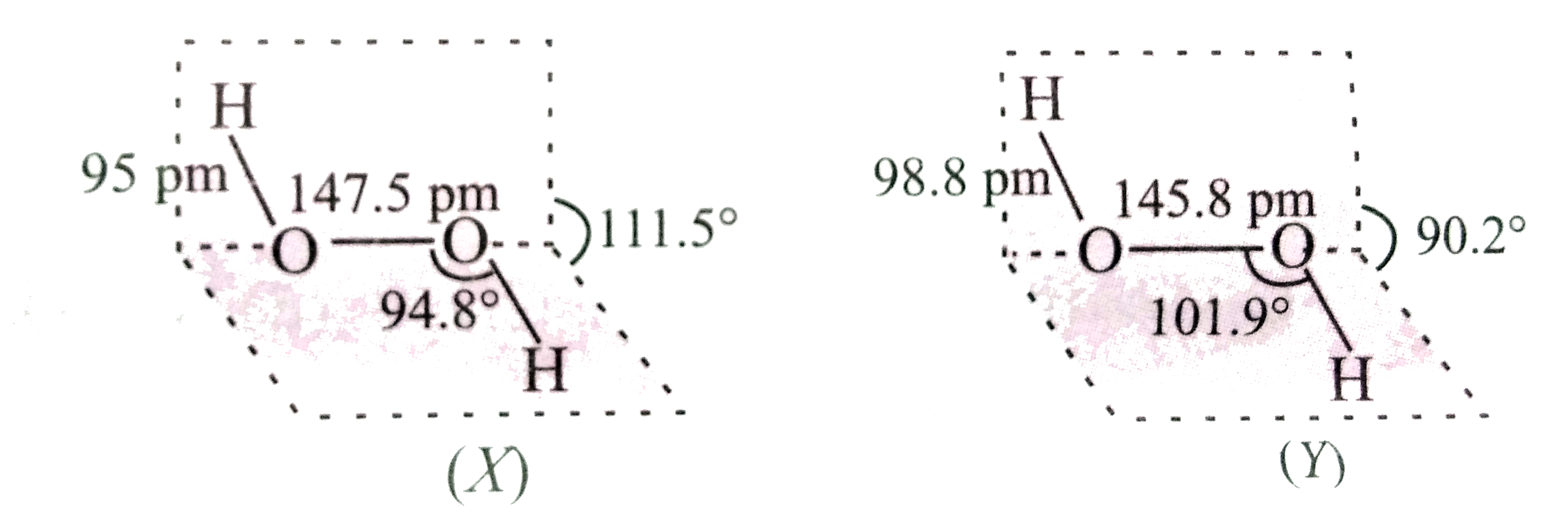A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise HOTS (HIGHER ORDER THINKING SKILLS)|9 VideosHYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise NCERT (EXEMPLAR PROBLEMS)|18 VideosHYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|12 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-HYDROGEN -Assertion And Reason
- Two structures of H(2)O(2) are drawn below. Identify the phases X and ...
Text Solution
|
- Assertion : In atomic form hydrogen consists of one proton and one ele...
Text Solution
|
- Assertion : Hydrogen resembles both, alkali metals as well as halogens...
Text Solution
|
- Assertion : All the three isotope of hydrogen have almost the same che...
Text Solution
|
- Assertion : Dihydrogen is inert at room temperature. Reason : The H ...
Text Solution
|
- Assertion : Hydrides of group 13 elements are Lewis acids whereas hydr...
Text Solution
|
- Assertion : Hydrides of N, O and F have lower boiling points than the ...
Text Solution
|
- Assertion : When sodium hydride in fused state is electrolysed, hydrog...
Text Solution
|
- Assertion : Ice cube floats on water. Reason : Density of ice is les...
Text Solution
|
- Assertion : CuSO(4).5H(2)O has one hydrogen-bonded molecule of water. ...
Text Solution
|
- Assertion : Soft water lathers with soap but not hard water. Reason ...
Text Solution
|
- Assertion : Permanent hardness of water can be removed by using washin...
Text Solution
|
- Assertion : In gaseous phase, H(2)O and H(2)O(2) both have bent struct...
Text Solution
|
- Assertion : A 30% solution of H(2)O(2) is marketed as '100 volume' hyd...
Text Solution
|
- Assertion : H(2)O(2) is stored in wax-lined glass or plastic vessels. ...
Text Solution
|
- Assertion : Melting and boiling points of D(2)O are higher than those ...
Text Solution
|