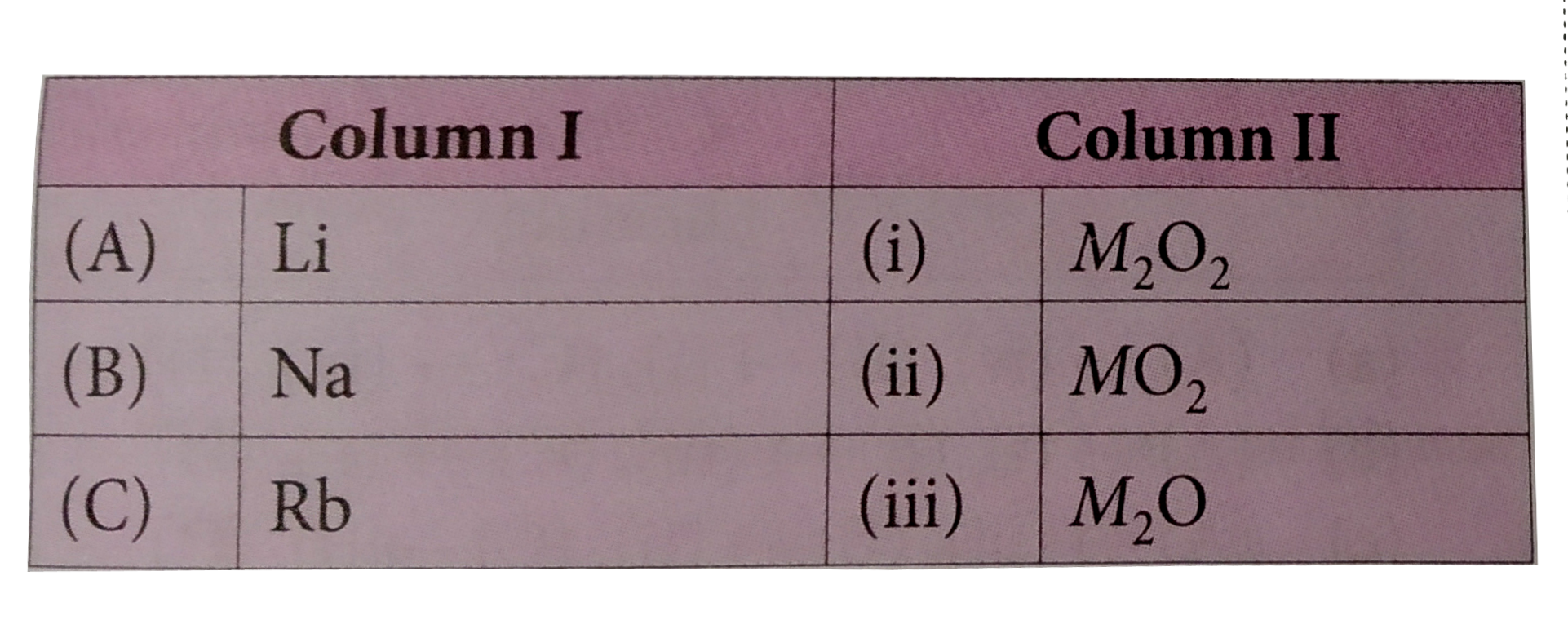
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise General Characteristic Of The Compounds Of The Alkali Metals|9 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Anomalous Properties Of Lithium|5 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion & Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE S-BLOCK ELEMENTS-Group 1 Elements - Alkali Elements
- The ionisation energy of alkali metals decreases from Li to Cs bacause
Text Solution
|
- First ionisation energy of alkali metals is very low but second ionisa...
Text Solution
|
- The solubility of alkli metals salts in water is due to the fact that ...
Text Solution
|
- The mobilities of the alkali metal ions in aqueous solution are Li^(+)...
Text Solution
|
- Arrange the following elements in the order of the increasing electrop...
Text Solution
|
- Which is the characteristic flame colouration of Li?
Text Solution
|
- Which of the following is not true about alkali metals?
Text Solution
|
- Which of the following alkali metals when burnt in air forms a mixture...
Text Solution
|
- A metal M reaccts with nitrogen to give nitride which on reaction with...
Text Solution
|
- Alkali metals cannot be extracted by reduction of their oxides and oth...
Text Solution
|
- When sodium reacts with excess of oxygen, oxidation number of oxygen c...
Text Solution
|
- In all oxides, peroxides and superoxides, the oxidation sate of alkali...
Text Solution
|
- Match column I wht colun II and mark the appropriate choice.
Text Solution
|
- The normal oxide containsion, peroxide containsion and superoxide cont...
Text Solution
|
- When sodium is added in scanty water, it catches fire. In this process...
Text Solution
|
- What happens when H(2) is passed over lithium at 1073K?
Text Solution
|
- On reaction with dihydrogen the alkali metals
Text Solution
|
- Lithium is the strongest reducing agent though it has highest ionisati...
Text Solution
|
- E^(@) for CL(2)//Cl^(-)=+1.36,I(2)//I^(-)=+0.53,Ag^(+)//Ag=+0.79,Na^(+...
Text Solution
|
- The alkali metals dissolve in ammonia to give a deep blue solution whi...
Text Solution
|