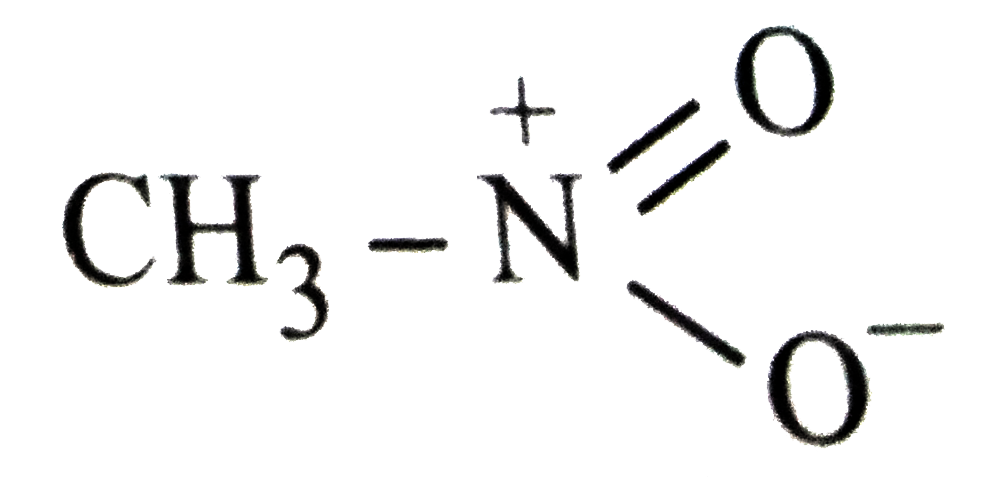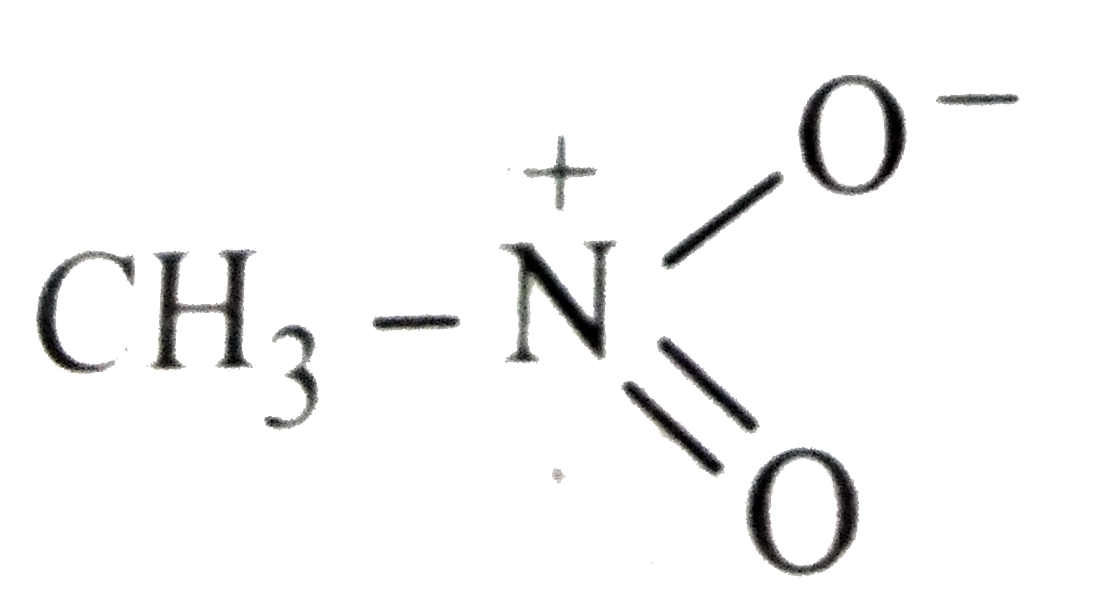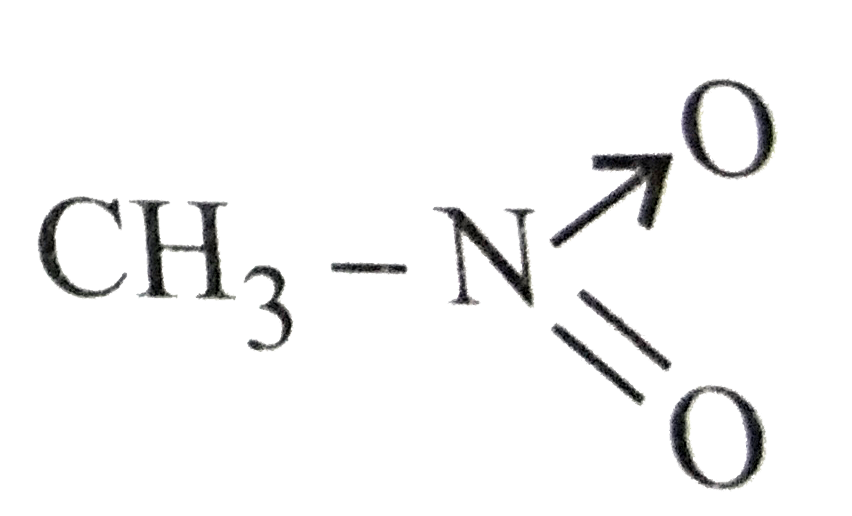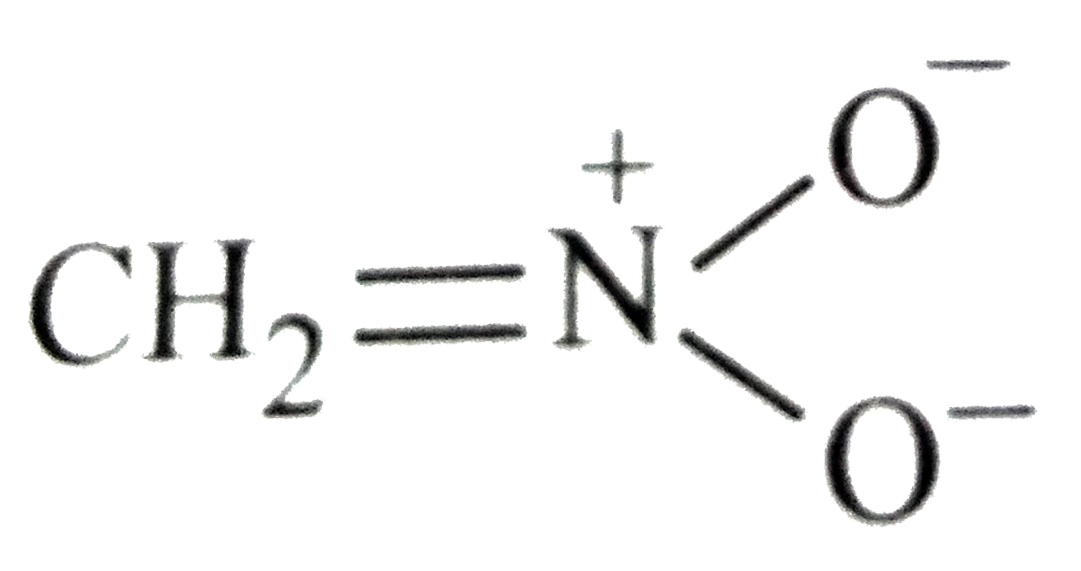A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Exemplar Problems|12 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Assertion & Reason|13 VideosHYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPRACTICE PAPER 1
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 1|44 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES -Assertion And Reason
- Which of the following is not structure of nitromethane molecule?
Text Solution
|
- Assertion: Hybridisation influences the bond length and bond enthalpy ...
Text Solution
|
- Assertion: sp^(3) hybrid carbon atom is more electronegative than sp h...
Text Solution
|
- Assertion: Rotation about C=C is restricted. Reason: Electron charge...
Text Solution
|
- Assertion: The name of the hybrocarbon (CH(3))(2)CHCH(2)CH(2)CH(CH(3...
Text Solution
|
- Assertion: Alkanes containing more than three carbon atoms exhibit cha...
Text Solution
|
- Assertion: Nitroalkanes and alkyl nitrites exhibit funcctional isomeri...
Text Solution
|
- Assertion: Heterolytic fission occurs readily in polar covalent bonds....
Text Solution
|
- Assertion: When inductive and electromeric effects operate in oppsite ...
Text Solution
|
- Assertionj: The following structures (I) and (II) canot e the major co...
Text Solution
|
- Assertion: The order of stability of carbocations is 3^(@) gt 2^(@) gt...
Text Solution
|
- Assertion: Glycerol is purified by distillation under reduced pressure...
Text Solution
|
- Assertion: Paper chromatography is a type of partition chromatography....
Text Solution
|