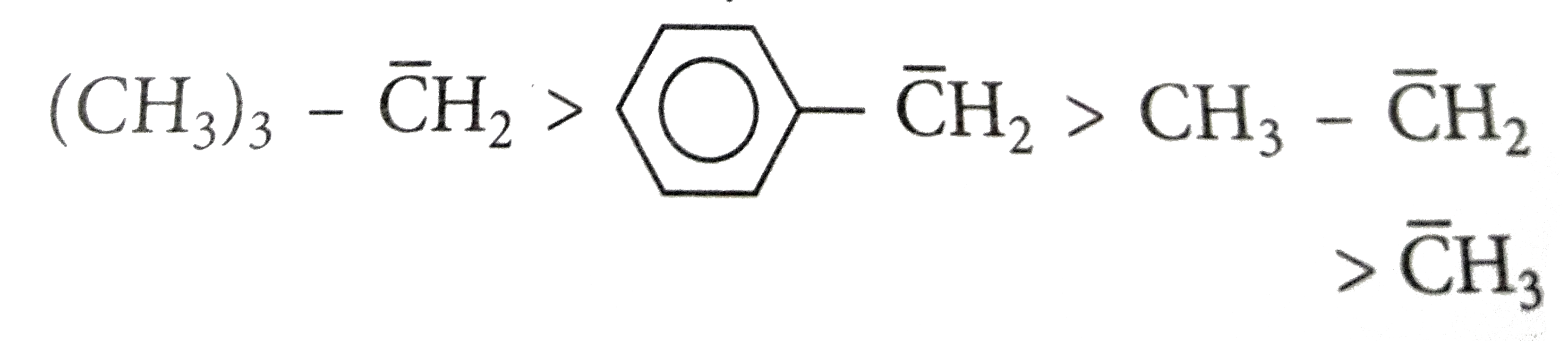
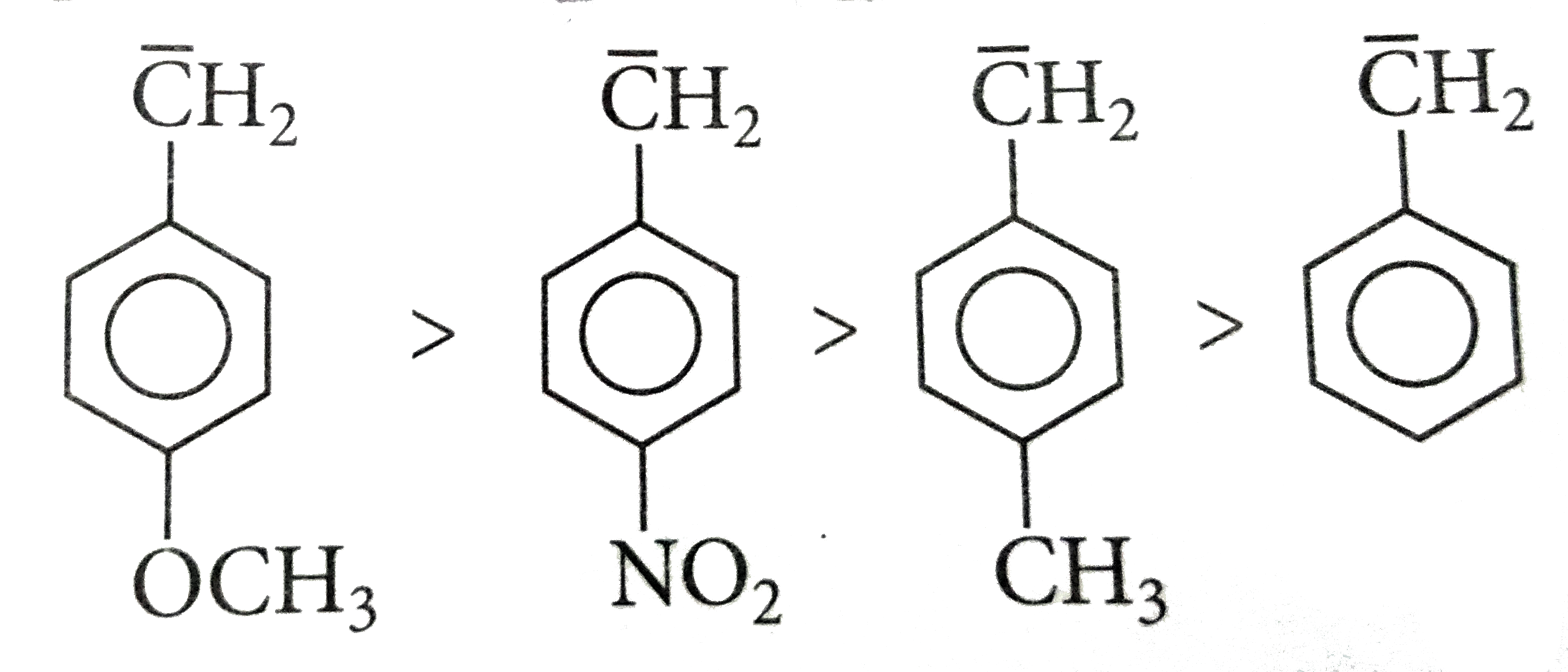
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|11 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|12 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Quantitative Analysis|11 VideosHYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPRACTICE PAPER 1
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 1|44 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES -Higher Order Thinking Skills
- Consider the following reactions. I. CH(2)=CHCOOH overset(Delta)to C...
Text Solution
|
- Which of the following names is correct for underset(CHO)underset(|)...
Text Solution
|
- The number of structural and configurational isomers of a bromo compou...
Text Solution
|
- Arrange the followinng carbocations in decreasing order of stability
Text Solution
|
- The correct stability order for the following species is
Text Solution
|
- Which of the following orders correctly depicts the decreasing order o...
Text Solution
|
- A sample of 0.50 g of an organic compound was treated according to Kje...
Text Solution
|